Research Article - (2024) Volume 13, Issue 5
HLA-DR, p16, p53 in Cervical Cancer in Southwester Nigeria
Kayode Adebowale Adelusola1*,
Ayodeji Olaonipekun Olutunde2,
Abiola Adeyemi Adefidipe3,
Norah Olubunmi Akinola4,
Adegoke Olaniyi Aremu3,
Ganiat Olutoyin Omoniyi-Esan1,
Adeleke Lukman Bisiriyu5 and
David Adesanya Ofusori6
1Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Anatomic Pathology, Federal Medical Center, Abeokuta, Nigeria
3Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria
4Department of Hematology and Immunology, Obafemi Awolowo University, Ile-Ife, Nigeria
5Department of Demography and Social Statistics, Obafemi Awolowo University, Ile-Ife, Nigeria
6Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile-Ife, Nigeria
*Correspondence:
Kayode Adebowale Adelusola, Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University, Ile-Ife,
Nigeria,
Tel: 2348033447433,
Email:
Received: 25-Sep-2024, Manuscript No. IPJBS-24-15222;
Editor assigned: 27-Sep-2024, Pre QC No. IPJBS-24-15222 (PQ);
Reviewed: 11-Oct-2024, QC No. IPJBS-24-15222;
Revised: 22-Oct-2024, Manuscript No. IPJBS-24-15222 (R);
Published:
29-Oct-2024
Abstract
Background: Cervical cancer remains the most common female genital tract malignancy, despite being preventable and possibly curative. The burden is enormous in resource-poor nations, where organized preventive screening methods are yet to be developed. Research efforts geared toward finding immunological and possibly therapeutic and prognostic markers are on-going and constitute the basis of this study.
Objectives: To determine the expression of the immune marker HLA-DR in cervical cancer patients as well as its possible associations with p16 and p53 in cervical cancer patients in Southwestern Nigeria.
Methods: Thirty and-eight cases of cervical cancer seen within a period of two years at two tertiary health institutions in Nigeria were processed for immunohistochemistry with HLA-DR, p53 and p16. Semi quantitative immunohistochemistry scoring was performed, and the results were analyzed via SPSS version 25. The expression of HLA-DR was correlated with that of p53 and p16, with the level of significance set at p<0.5. Pearson correlation analysis of independent variables was performed.
Results: The peak age of cervical cancer incidence was 50-59 years. Thirty patients had squamous cell carcinoma. High and moderate expression of HLA-DR was observed in 23.7% of the patients, 28.9% of the p16 patients and 7.9%of the p53 patients. There was no relationship between HLA-DR expression and age (r=-0.23, p=0.159), or p16 (r=-0.159, p=0.340), but there was a strong negative relationship with p53 (r=0.92, p=0.581).
Conclusion: Among the three markers used, p16 was most strongly associated with cervical cancer, followed by HLA-DR and p53. HLA-DR is likely not a reliable biological marker of cervical cancer and may not be a useful therapeutic target in cervical cancer.
Keywords
Cervical cancer; HLA-DR; p16; p53; Nigeria
Introduction
Cervical Cancer (CC) is the fourth most common malignancy in
females and the most common female genital tract malignancy
worldwide. Globally, an estimated 604,127 new cases of CC and
341,831 CC-related deaths occurred in 2020. Although the
incidence has decreased in some countries, increasing incidence
has been observed in many parts of sub-Saharan Africa [1]. In
middle and low-income countries of sub-Saharan Africa, where
the incidence is high, a lack of effective prevention and
treatment strategies as well as the burden of Human
Immunodeficiency Virus (HIV) infection may worsen the
prognosis [2]. A recent study revealed that most women are
unaware of CC, risk factors and symptoms of the disease [3].
Whereas routine screening for precancerous cervical lesions has
led to a decrease in the incidence of CC in developed nations, in
many low-income countries, the national screening program for
CC has not been successfully implemented. CC has been strongly
associated with persistent HPV infection worldwide, although
there are other risk factors and co-factors involved in cervical
carcinogenesis. Attention is currently being focused on the
possible role of Human Leukocyte Antigen (HLA) in
immunosurveillance and as a candidate tumor suppressor in
malignancies [4]. HLA, the expression product of the human
MHC, is located on chromosome 16p21.31. Class II antigens are
constitutively expressed on professional antigen-presenting cells such as B-lymphocytes, dendritic cells, macrophages,
monocytes, Langerhans cells and endothelial cells. Many cell
types, including some tumors, are also capable of expressing
class II MHC, an expression that may determine the prognosis in
some cases [5]. There is evidence that some HLA class II alleles
may be involved in CC [6].
P16 (CDKN2A) is a member of the INK4 cell cycle inhibitor
family. It is a tumor suppressor protein, and a CDK inhibitor
essential for regulating the cell cycle [7]. P16 is considered a
surrogate marker for HPV-related head and neck squamous cell
carcinomas. de Wispelaere, et al., in an analysis of 124 different
tumor types by IHC, noted that the highest p16 positivity rates
(94.4%) were in Squamous Cell Carcinoma (SCC) of the cervix.
HPV was noted in 80.4% of p16-positive and 20.6% of p16-negative cancers [8]. They concluded that p16 may not be a
surrogate marker for HPV except in tumors of the cervix and
penis.
The p53 gene is another tumor suppressor gene and cell cycle
regulator; it is the guardian of the genome and is encoded by
TP53 on chromosome 17. Loss of wild-type p53 activity leads to
deregulation of the p53 signaling pathway. p53 is mutated in
50% of human cancers. This involves inactivation of its pathway,
including MDM2 amplification, loss of p14ARF and mutations in
activating kinases such as ATM and Chk2. Loss of p53 function
gives cancer cells a survival advantage to bypass the resolution
of oncogenic signals and DNA damage to continue proliferation
[9]. This study investigated the expression of the immune
markers HLA-DR as well as, p16 and p53 in CC and the
relationships between these markers in CC with the goal of
contributing to the literature on the biology of CC.
Materials and Methods
Thirty eight anonymized cases of CC out of forty cases seen
within a period of two years (January 2022-December 2023) at
two tertiary health institutions in two states in southwestern
Nigeria, the Obafemi Awolowo University Teaching Hospitals
Complex (OAUTHC) in Ile-Ife, Osun State, and the Federal
Medical Center, Abeokuta, in Ogun State, were included in this
study. The tissues were fixed in formalin and embedded in
paraffin. Immunohistochemical staining was performed on 4 μm
thick deparaffinized sections with HLA-DR (Clone MA5 5-11966
from Thermo-Fisher Scientific), p16 (Clone 6154652 from BD
Pharmingen) and p53 (Clone MS 18P DO-7 from Epredia) via the
indirect immunoperoxidase and avidin-biotin method and
TA-125-ADQ antibody diluent (Thermo Fisher Scientific).
Appropriate positive and negative controls were used. Semiquantitative
IHC scores were obtained via multiplication of the
intensity of nuclear or cytoplasmic staining (0-3 where 0
indicates no staining, 1-indicates weak, 2-indicates moderate
and 3-indicates strong staining) by the score of the percentage
of positively stained cells (0-30-3 where 0 indicates ≤ 5%, 1
indicates 6%-25% positive cells, 2-26%-50% positive cells and 3
≥ 51% positive cells). Protein expression was considered low if
the product of the staining intensity and percentage of stained
tumor cells score was ≤ 3 and high if the product was ≥ 4.25.
Negative expression was assigned a score of 0. The expression
pattern of HLA-DR was correlated with p16 and p53 scores via
SPSS Version 25. The level of significance was set at p<0.05.
Results
The ages ranged from 35 years to 83 years (mean 52.3 years;
SD 12.1). Thirty two patients (84.2%) had squamous cell
carcinoma (nineteen with keratinized cancer and thirteen
without keratinized cancer). The remaining patients had four
adenocarcinomas, one each of transitional cell carcinoma and
poorly differentiated carcinoma. Table 1 shows the percentage
frequency by age group.
| Variable |
Frequency |
Percentage |
| Age groups (in years) |
| 30–39 |
5 |
13.1 |
| 40–49 |
8 |
21.1 |
| 50–59 |
15 |
39.5 |
| 60–69 |
6 |
15.8 |
| ≥ 70 |
4 |
10.5 |
Table 1: (N=38) percentage by age group.
The categories of the degree of positivity for each antibody
are shown in Figure 1. P16 had the highest degree of positivity in terms of moderate and high expression. Figure 2 shows the
various intensities of antibody staining.
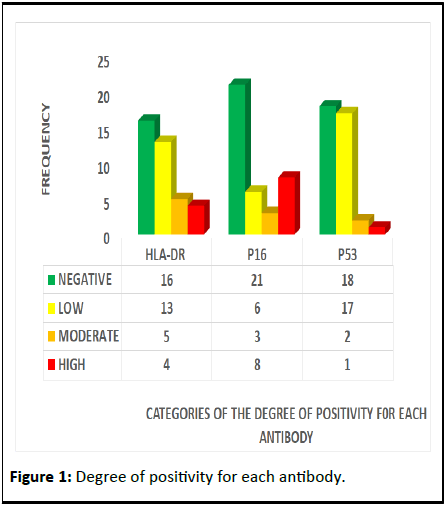
Figure 1: Degree of positivity for each antibody.
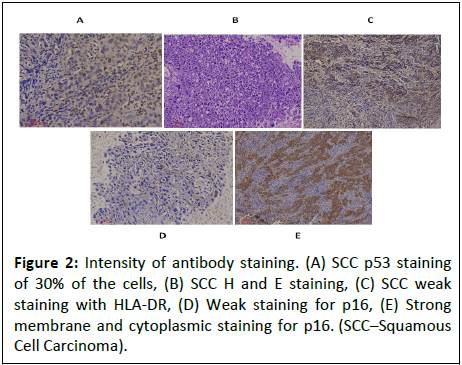
Figure 2: Intensity of antibody staining. (A) SCC p53 staining
of 30% of the cells, (B) SCC H and E staining, (C) SCC weak
staining with HLA-DR, (D) Weak staining for p16, (E) Strong
membrane and cytoplasmic staining for p16. (SCC–Squamous
Cell Carcinoma).
Pearson correlation analysis revealed that there was no
relationship between HLA-DR independent variables, such as
age (r=-0.23, p=0.159), or p16 (r=-0.159, p=0.340), but there was
a strong negative relationship with p53 (r=0.92, p=0.581) (Table
2).
| Variables |
Correlation statistics with HLA-DR |
| Correlation coefficient |
p value |
| Age (in years) |
-0.233 |
0.159 |
| p16 Score |
-0.159 |
0.34 |
| p53 Score |
0.92 |
0.581 |
Table 2: Associations between HLA-DR and the independent variables (age and p16 and p53 scores) via Pearson correlation analysis (n=38).
Discussion
Despite being preventable through routine screening, early
detection and HPV vaccination, morbidity and mortality from
cervical cancer still constitute an enormous burden globally,
mostly in resource limited nations. The incidence and mortality
of this disease have drastically decreased in high income nations
as a result of effective primary prevention strategies. Some
cases of CC in the developing world do not present at the
hospital before death or are seen but cannot afford the cost of
preliminary investigations and treatment. Therefore, cases of CC
in low-income countries may be grossly underreported and
therefore underestimated. The peak age incidence of 50-59
years in this study is a welcome departure from the 40-49 years
reported in a study [10] from One of the Two Centers (OAUTHC)
in 2004, two decades earlier than the present study. A peak age
of 40-69 years, a rather wide age range, was reported in another
study from another part of Nigeria [11]. Only 26.3% of CCs had
moderate or high expression of HLA-DR in this study. Madeleine
and Brumback [12], in their study of 315 patients, demonstrated
that some HLA-DR alleles are associated with an increased risk
of SCC of the cervix, whereas other alleles or allele combinations
confer a low risk. These findings underscore the importance of
molecular studies using different HLA alleles in the risk
stratification of CC. A study by Chambuso, et al., [13] also
revealed an association of some HLA-DR alleles with cervical
cancer patients with concurrent HIV infection, an association
that was rare or absent in females with non-malignant cervical
disease. It is not known how many of the patients in this study
had concurrent HIV infection or other sexually transmitted viral infections, which may be relevant in CC carcinogenesis. Unless
suspected or indicated, routine screening for HIV is not
performed for CC patients in our study centers. The role of
herpes simplex virus type II in cervical cancer is controversial at present [14]. In the study by Musa et al in North Central Nigeria,
8.2% of patients with CC tested positive for HIV [15]. The
increased expression of HLA genes has been found to be
associated with prolonged survival in patients with tumors
studied by Shaafsma, et al. [16]. Samuels, et al., concluded that
the upregulation of HLA-DRA is significantly related to increased
disease-free survival and disease survival in patients with
cervical adenocarcinoma [17].
However, that observation may have limited application in
this study since the majority of our patients had squamous cell
carcinoma. P53 was strongly negatively associated with HLA-DR
in this study. Frier, et al., [18] reported that 66% of cancers of
the cervix studied expressed p53. The p53-positive patients also
had better survival, implying a better response to chemotherapy.
The role of p53 loss in cancer cells is enormous. It enables
cancer progression and presumably affects the response of
cancer cells to different chemotherapeutic regimens [19]. While
p53 is important in preventing cancer, inappropriate p53
activation can also have detrimental effects by promoting
various pathological states and developmental phenotypes [20].
A balanced p53 activity is therefore imperative in the
development of p53-based therapy. The overexpression of p53 is
a significant prognostic factor in luminal/HER2-negative breast
cancer [21]. No comparable finding has been noted in CC.
Various therapeutic strategies targeting mutant p53 (mutp53)
and unresolved obstacles facing mutp53 targeted therapy for
cancer have been described by Zhang, et al. [22]. Despite these
uncertainties, p53 is still an important determinant of cell fate in
response to chemotherapy, under appropriate treatment
conditions [23]. The low expression of p53 in this study likely
implies that the tumors are highly malignant and may not have a
good response to therapy. Most cases in Nigeria, as in many
resource-limited countries, present late to the hospital at
advanced disease stages for many reasons. In Lagos, for
example, 72.81% of cervical cancer patients are seen in hospitals
at late stages. The reasons for this include fear, misconception,
sometimes misdiagnosis, ignorance and prolonged investigation
time [24]. The same pattern is recorded in some parts of Nigeria,
such as Zaria at 78% [11], North Central China at 72.3% [15] and
Ghana at 95% [25]. Financial constraints and distance to a
healthcare facility are some of the reasons adduced for this. This
study revealed the expression of HLA-DR in cervical cancer. With
the use of different HLA alleles, a clearer picture may emerge.
No significant associations of p16 or p53 with cervical cancer or
HLA-DR were detected in this study. The implications of these
findings with respect to CC biology, chemotherapy,
immunotherapy and prognosis are not clear. Okonofua, et al.
[26], in a recent editorial, emphasized the prioritization of
primary prevention of CC with the HPV vaccine as a more cost-effective
method than secondary and tertiary prevention
strategies. Fortunately, HPV vaccination has taken a foothold in
many African countries. The African populace must be
enlightened as to the usefulness and efficacy of the vaccine in
view of the numerous conspiracy theories of directed at the
vaccine.
Conclusion
P16 was expressed at the highest level in cervical cancer,
followed by HLA-DR and p53. Further studies using different
HLA-DR alleles are recommended. Primary prevention of cervical
cancer with HPV vaccination is most appropriate in resourcelimited
countries.
Ethics Approval
National ethics approval No NHREC/ 01/01/2007-24/10/2019.
Consent to Participate
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
Available on upon request from the corresponding author.
Funding
This study was sponsored by the Tertiary Education Trust
Fund (TETFund) of the Federal Government of Nigeria.
Authors' Contributions
KAA, NOA, GOO, and DAO initiated the study. KAA wrote the
manuscript. AOO contributed cases from his center. AAA and
AOA were responsible for the histopathology and
immunohistochemistry aspects of the study. ALB performed the
statistical analysis. NOA is shown in the bar chart. All the authors
read the completed manuscript.
Acknowledgment
Dr. Olaejirinde Olaofe for additional statistical analysis.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, et al. (2023) Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health 11:e197-206
[Crossref] [Google Scholar] [PubMed]
- Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, et al. (2021) Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 9:e161-e169
[Crossref] [Google Scholar] [PubMed]
- Olubodun T, Balogun MR, Odeyemi AK, Odukoya OO, Ogunyemi AO, et al. (2022) Barriers and recommendations for a cervical cancer screening program among women in low-resource settings in Lagos Nigeria: A qualitative study. BMC Public Health 22:1906
[Crossref] [Google Scholar] [PubMed]
- Pujadas E, Cordon-Cardo C (2021) The human leukocyte antigen as a candidate tumor suppressor. Cancer Cell 39:586-589
[Crossref] [Google Scholar] [PubMed]
- Forero A, Li Y, Chen D, Grizzle WE, Updike KL, et al. (2016) Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol Res 4:390-399
[Crossref] [Google Scholar] [PubMed]
- Zhao M, Qiu L, Tao N, Zhang L, Wu X, et al. (2013) HLA DRB allele polymorphisms and risk of cervical cancer associated with human papillomavirus infection: A population study in China. Eur J Gynaec Oncol 28:2007
[Google Scholar] [PubMed]
- LaPak KM, Burd CE (2014) The molecular balancing act of p16INK4a in cancer and aging. Mol Cancer Res 12:167-183
[Crossref] [Google Scholar] [PubMed]
- de Wispelaere N, Rico SD, Bauer M, Luebke AM, Kluth M, et al. (2022) High prevalence of p16 staining in malignant tumors. PLoS One 17:e0262877
[Crossref] [Google Scholar] [PubMed]
- Borrero LJ, El-Deiry WS (2021) Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. BBA Rev Cancer 1876:188556
[Crossref] [Google Scholar] [PubMed]
- Omoniyi-Esan G, Fasubaa O, Adelusola K, Ojo O (2004) Histological pattern of cervical malignancy in Southwestern Nigeria. Trop J Obstet Gynaecol 21:118-122
- Oguntayo OA, Zayyan M, Kolawole AO, Adewuyi SA, Ismail H (2011) Cancer of the cervix in Zaria, Northern Nigeria. Ecancer Med Sci 5
[Crossref] [Google Scholar] [PubMed]
- Madeleine MM, Brumback B, Cushing-Haugen KL, Schwartz SM, Daling JR, et al. (2002) Human leukocyte antigen class II and cervical cancer risk: A population-based study. J Infect Dis 186:1565-1574
[Crossref] [Google Scholar] [PubMed]
- Chambuso R, Ramesar R, Kaambo E, Denny L, Passmore JA, et al. (2019) Human Leukocyte Antigen (HLA) Class II-DRB1 and-DQB1 alleles and the association with cervical cancer in HIV/HPV co-infected women in South Africa. J Cancer 10:2145
[Crossref] [Google Scholar] [PubMed]
- Sausen DG, Shechter O, Gallo ES, Dahari H, Borenstein R (2023) Herpes Simplex virus, human papillomavirus, and cervical cancer: Overview, relationship, and treatment implications. Cancers 15:3692
[Crossref] [Google Scholar] [PubMed]
- Musa J, Nankat J, Achenbach CJ, Shambe IH, Taiwo BO, et al. (2016) Cervical cancer survival in a resource-limited setting-North Central Nigeria. Infect Agent Cancer 11:1-7
[Crossref] [Google Scholar] [PubMed]
- Schaafsma E, Fugle CM, Wang X, Cheng C (2021) Pan-cancer association of HLA gene expression with cancer prognosis and immunotherapy efficacy. Br J Cancer 125:422-432
[Crossref] [Google Scholar] [PubMed]
- Samuels S, Spaans VM, Osse M, Peters LA, Kenter GG, et al. (2016) Human leukocyte antigen-DR expression is significantly related to an increased disease-free and disease-specific survival in patients with cervical adenocarcinoma. Int J Gynecol Cancer 26
[Crossref] [Google Scholar] [PubMed]
- Freier CP, Stiasny A, Kuhn C, Mayr D, Alexiou C, et al. (2016) Immunohistochemical evaluation of the role of p53 mutation in cervical cancer: Ser-20 p53-mutant correlates with better prognosis. Anticancer Res 36:3131-3137
[Google Scholar] [PubMed]
- Marcus JM, Burke RT, Doak AE, Park S, Orth JD (2018) Loss of p53 expression in cancer cells alters cell cycle response after inhibition of exportin-1 but does not prevent cell death. Cell Cycle 17:1329-1344
[Crossref] [Google Scholar] [PubMed]
- Wang M, Attardi LD (2022) A balancing act: p53 activity from tumor suppression to pathology and therapeutic implications. Annu Rev Pathol 17:205-226
[Crossref] [Google Scholar] [PubMed]
- Kikuchi S, Nishimura R, Osako T, Okumura Y, Nishiyama Y, et al. (2013) Definition of p53 overexpression and its association with the clinicopathological features in luminal/HER2-negative breast cancer. Anticancer Res 33:3891-3897
[Google Scholar] [PubMed]
- Zhang C, Liu J, Xu D, Zhang T, Hu W, et al. (2020) Gain-of-function mutant p53 in cancer progression and therapy. J Mol Cell Biol 12:674-687
[Crossref] [Google Scholar] [PubMed]
- Lukin DJ, Carvajal LA, Liu WJ, Resnick-Silverman L, Manfredi JJ (2015) p53 Promotes cell survival due to the reversibility of its cell-cycle checkpoints. Mol Cancer Res 13:16-28
[Crossref] [Google Scholar] [PubMed]
- Awofeso O, Roberts AA, Salako O, Balogun L, Okediji P (2018) Prevalence and pattern of late stage presentation in women with breast and cervical cancers in Lagos University Teaching Hospital, Nigeria. Nigerian Med J 59:74-79
[Crossref] [Google Scholar] [PubMed]
- Appiah-Kubi A, Konney TO, Amo-Antwi K, Tawiah A, Nti MK, et al. (2022) Factors associated with late-stage presentation of cervical cancer in Ghana. Ghana Med J 56:86-94
[Crossref] [Google Scholar] [PubMed]
- Okonofua F, Edouard L, Isikhuemen M (2023) Human Papilloma Virus (HPV) vaccine and cervical cancer prevention in Africa. Afr J Reprod Health 27:9-14
[Crossref] [Google Scholar] [PubMed]
Citation: Adelusola KA, Olutunde AO, Adefidipe AA, Akinola NO, Aremu AO, et al. (2024) HLA-DR, p16 and p53 in Cervical Cancer in Southwestern Nigeria. J Biomed Sci Vol:13 No:5







