Key words
|
| |
| Linum usitatissimum L; Ether insoluble phenolic components from n-butanol fraction (EPC-BF); Phenolic acid; Caffiec acid; HPTLC; LC-ESI-MS. |
| |
Introduction
|
| |
| Linum usitatissimum L. belongs to family Linaceae, commonly known as linseed or flaxseed. Since, antiquity it has been cultivated worldwide for oil and fiber. Flaxseed is the richest source of omega- 3 fatty acid, A-linolenic acid (ALA), fiber and mucilage. Besides this, it is also the worthy source of various phenolic compounds such as lignan, phenolic acids, flavonoids etc. Flaxseed contains lignan secoisolariciresinol diglucosides (SDG), SDG isomer, matairesinol, pingoresinol diglucoside and isolariciresinol. Phenolic acids such as p- coumaric, o- coumaric, ferulic, p- hydroxybenzoic, gentisic, vanilic, sinapic acids in free and /or bound form were reported in flaxseed [1, 2]. Caffiec acid and their glucosides were reported in the flaxseed as well [3]. Furthermore, flaxseed reported to contain phenylpropanoid glucoside; linusitamarin, linocinamarin and daucosterol along with variety of flavonoids such as herbacetin 3,8-Odiglucopynanoside, herbacetin 3, 7-O-dimethyl ether, kaempferol 3,7-O-diglucopyranoside and herbacetin diglucoside [4]. |
| |
| High performance thin layer chromatography (HPTLC) and the coupling of mass spectrometry with liquid chromatography (LC/MS) are becoming important online tools for the characterization and identification of compounds in crude plant extract. In previous study, we reported a simple novel approach for selective isolation of ether insoluble phenolic components from n-fraction (EPC-BF) of defatted flaxseed meal and studied their antioxidant potential [5]. In the current study, HPTLC and LC-ESI-MS methods were used to characterize EPC-BF from defatted flaxseed meal. |
| |
Material and Methods
|
| |
| Chemicals and reagents |
| |
| Methanol, n-hexane, n-butanol, solvent ether, Folin- Ciocalteu reagent were purchased from Molychem (Mumbai, India). Water for HPLC and Silica gel 60 F254 were purchased from Merck (India). All these chemicals and reagents were of analytical grade. |
| |
| Defatted flaxseed meal and extraction of EPC-BF A double pressed flaxseed cake powder obtained from “Omega-3 oil unit, under project NAIP-ICAR Component-3, Sangamner, M.S., India” was used for extraction. The extraction of EPC-BF was carried out by as per our previously described method [5]. Briefly, Residual oil from flaxseed meal was removed by n-hexane (1:4, w/v). 1 Kg dried defatted flaxseed powder was extracted with methanol for 3 hrs at 60 oC in a soxhlet apparatus. Methanol extract was concentrated in rotavapour at 60°C under vacuum. Then the dried residue further partitioned with nbutanol: water (1:1, v/v). n-Butanol fraction was separated from aqueous fraction and concentrated in rotavapour at 80°C under vacuum. Dried n-butanol residue was dissolved in minimum quantity of methanol and precipitated with solvent ether (1:5, v/v), yields brown colored sticky precipitate of EPCBF. Finally, EPC-BF was dissolved in methanol and after syringe filter used for HPTLC and LC-ESI-MS characterization. |
| |
| HPTLC characterization of EPC-BF |
| |
| HPTLC profile of EPC-BF was prepared with use of a CAMAG HPTLC system. Silica gel plate (Silica gel 60 F254, Merck) was used for HPTLC analysis. 2 μl of EPC-BF was applied onto the plates as 6 mm band, 8 mm from the bottom edge by means of a 50 μL Hamilton microsyringe. The separation was carried by using solvent system butanol: water (5:4, v/v, upper layer). The chromatogram was developed in glass twin-trough chamber (10 cm × 10 cm, with metal lids; CAMAG, Switzerland) previously saturated with mobile phase vapor for 20 min. The development distance was 70 mm. After development, the plate was dried with dryer. Chromatogram was scanned from 190-700 nm to get λmax and chromatogram was developed at 310 nm. Finally, the HPTLC plate was derivatized with Folin- Ciocalteu reagent. |
| |
| Liquid chromatography electrospray ionization mass spectrometer analysis (LC-ESI-MS) of EPC-BF |
| |
| The EPC-BF in HPLC grade methanol (~ 1mg/ml) was analyzed on Varian 500-MS liquid chromatography mass spectrometry at 310 nm. The separation was carried on C18 column (pursuit 5u, 100 x 2.0 mm, Varian) with flow rate 0.5 ml/min. Isocratic mobile phase methanol: water (65:35, v/v) was used. The ESI-MS conditions were; air was used as nebulizer/drying gas. Drying gas temperature was set at 350oC with nebulizer pressure of 50 psi. needle voltage was set at -5000V. Spreyshield voltage was set at -600 V. Mass spectra were obtained in negative ionization mode over m/z 100-1500. |
| |
Result and discussion
|
| |
| In earlier study, we selectively isolated ether insoluble phenolic components (EPC-BF) from nbutanol fraction; free from earlier reported lignan SDG, phenylpropanoid and flavonoids in n-butanol fraction of defatted flaxseed meal and studied their in vitro antioxidant potential. In addition, UV spectroscopic characterization and preliminary phytochemical screening of EPC-BF were also carried out; results of this study indicated only presence of phenolic acids, tannins and carbohydrates. |
| |
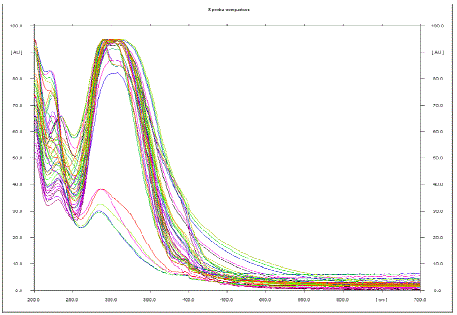
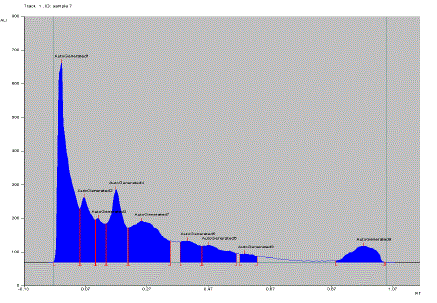
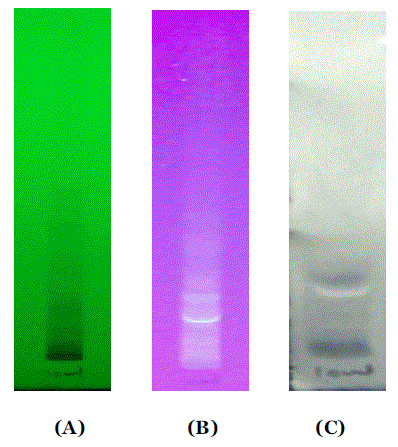
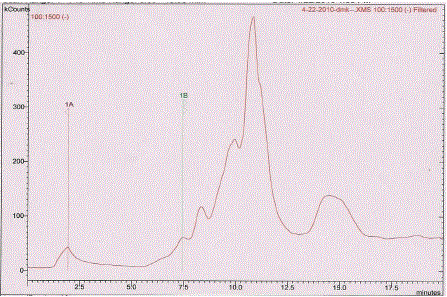
| Here, to support and to prove our previous findings, to identified phenolic components, further characterization of EPC-BF was carried by HPTLC and LC-ESI-MS. HPTLC is the simplest and rapid method for separation and quantification of phytoconstituents. The results of HPTLC are more accurate and reproducible than simple TLC. In combination with digital scanning profiling, HPTLC also provides accurate and precise Rf values and quantitative analysis of sample constituents [6]. Fig. 1 shows the scanning spectra of EPC-BF, scanned from 190 to 700 nm. HPTLC scanning spectra of EPC-BF exhibited maximum absorption within range 290- 310 nm, which supports to the results of our earlier studied UV spectra of EPC-BF [5]. Fig. 2 reveals HPTLC chromatogram of EPC-BF. HPTLC chromatogram of EPC-BF indicated presence of nine peaks with the characteristic Rf value 0.07, 0.11, 0.17, 0.26, 0.3, 0.41, 0.51, 0.6, 0.97 respectively. Similarly, the maximum absorption of individual assigned peak was found to 309, 309, 295, 307,309, 290, 308, 200, 200 nm respectively. To confirm the presence of phenolic acid especially phenolic acid with catechol or hydroquinone nuclei in EPC-BF, derivatization of plate with Folin-Ciocalteu reagent was done. Fig. 3 shows results of HPTLC chromatographic plate under UV and after derivatization with Folin- Ciocalteu reagent. Appearance of blue colored spots after derivatization with Folin-Ciocalteu reagent confirmed the presence of phenolic acids in EPC-BF. LC-MS seemed to be especially useful in speculating structures of trace amounts of natural compounds when standard compounds are not available. LC-ESIMS analysis of EPC-BF was carried out to identify phenolic components of EPC-BF. Table.1 shows the retention time (tR), base peak m/z and tentative identification of base peak based on mass spectral data and published literature reports. The results of LC-ESI-MS analysis of EPC-BF (Fig. 4) indicated six different peaks with base peak m/z 503.3, 1341.2, 549.2, 179.1, 623.1 and 311.2 respectively. Peak 4, which showed base peak [M-H]- ion at m/z 179.1, was tentatively identified as of caffeic acid. Since, caffiec acid reported to has m/z at 179.1 [7]. On the basis of molecular weight, peaks 1 with m/z 503 and peak 3 with 549.2 with could be of prototannin and peak 2 with m/z 1304 could be of hydrolysable tannin [8]. Peak 5 showed base peak [M-H]- ion at m/z 623.1 could be of aceteoside 1 or 2 [9] or was iosrhamnetic diglucosides [10]. Peak 6 with m/z 311 tentatively identified as 3-formyl-4, 6-dihydroxy-2- methoxy-5-methylcalcone [11]. However, further detail spectroscopic characterization of EPC-BF through MS, IR, and NMR is essential to support our findings. |
| |
|
Conclusion
|
| |
| HPTLC and LC-ESI-MS characterization of EPC-BF tentatively confirmed the presence of phenolic acids such as caffeic acid, prototannin, hydrolysable tannin in EPC-BF of defatted flaxseed meal along with phenolic glycosides. However, we suggest further purification and characterization individual phenolic components from EPC-BF of flaxseed are essential to support our findings. |
| |
Acknowledgements
|
| |
| The authors are grateful to IIT Bombay, Mumbai for LC-ESI-MS facility. |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










