Keywords
|
| |
| Polyphenols, antioxidant activity, reactive oxygen species, HPTLC, Melothria heterophylla and radical scavenging |
| |
INTRODUCTION
|
| |
| Free radicals which are generated either exogenously or endogenously inside the body have been implicated in the causation of several disorders such as liver cirrhosis [1], inflammation, atherosclerosis [2], diabetes, cancer [3] and neurodegenerative disease [4]. The linkage between the free radicals and disease processes has led to considerable research of nontoxic drugs that can scavenge the free radicals. Several plant extracts and plant products have been shown to possess significant antioxidant potential [5-6]. Melothria heterophylla (Lour.) Cogn (Family- Cucurbitaceae) popularly known as kudari is a scandent herb with tuberous roots found throughout India ascending upto 2,100 m in the hills. They are considered to be used by the tribals of Orissa for their stimulant, invigorating and purgative property [7-8]. The juice of leaves is applied to the parts inflamed by the application of the marking nut juice (from Semecarpus anacardium Linn). In fact, there is only a single report on the antioxidant activity of Melothria heterophylla Lour. Cogn extracts [9], and in this report we have done a systematic investigation on the antioxidant potential of ethyl acetate, ethanolic and aqueous extracts of the plant in vitro. |
| |
MATERIALS AND METHODS
|
| |
|
Plant material
|
| |
| The plant Melothria heterophylla (Lour.) Cogn., was identified by Botanical Survey of India, Shibpur, Howrah, India. A voucher specimen has been preserved in our laboratory for future reference (AD-1). After authentication, fresh aerial parts were collected in bulk from young matured plants from Mayurbhanj District of North Orissa, washed, shade dried and then milled to course powder by a mechanical grinder. |
| |
| Chemicals |
| |
| 1,1-diphenyl-2-picryl-hydrazyl (DPPH), butylated hydroxy toluene (BHT), Curcumin, ascorbic acid, sodium nitroprusside, nicotinamide adenine dinucloetide (NADH), nitrobluetetrazolium (NBT), phenazine methosulphate, sulphanilamide, naphthylethylene diamine dihydrochloride and potassium ferricyanide were purchased from Sigma Chemical Co. Ltd, USA. All other chemicals and reagents were of analytical grade. |
| |
| Preparation of the extract |
| |
| The powdered plant material was extracted in succession with petroleum ether (60-80°C), chloroform, ethyl acetate, ethanol and distilled water using soxhlet apparatus. The solvent was then removed under reduced pressure, which gave colored residues for petroleum ether (PEMH), chloroform (CEMH), ethyl acetate (EAMH), ethanol (EEMH) and aqueous extract (AEMH) respectively. The completely dried individual extracts were used for evaluation of in-vitro antioxidant activity. |
| |
| Phytochemical screening |
| |
| The extracts were screened for the presence of various constituents employing standard screening test [10]. Other conventional protocols were also used for detecting the presence of steroids, alkaloids, tannins, flavonoids, glycosides, etc. |
| |
| Determination total polyphenolic compounds |
| |
| The concentrations of phenolic content in all the fractions were determined with Folin–Ciocalteu’s phenol reagent (FCR) according to the method of Slinkard and Singleton [11]. 1 ml of the sample solution (contains 1 mg) of the extract/fractions in methanol was added to 46 ml of distilled water and 1 ml of FCR, and mixed thoroughly. After 3 min, 3 ml of sodium carbonate (2%) were added to the mixture and shaken intermittently for 2 h at room temperature. The absorbance was measured at 760 nm. The concentration of phenolic compounds was calculated according to the following equation that was obtained from standard pyrocatechol graph (R2 = 0.9965): |
| |
| Absorbance = 0.001pyrocatechol (μg) + 0.0033 |
| |
| HPTLC Analysis of Phenolic and Antioxidant Substances |
| |
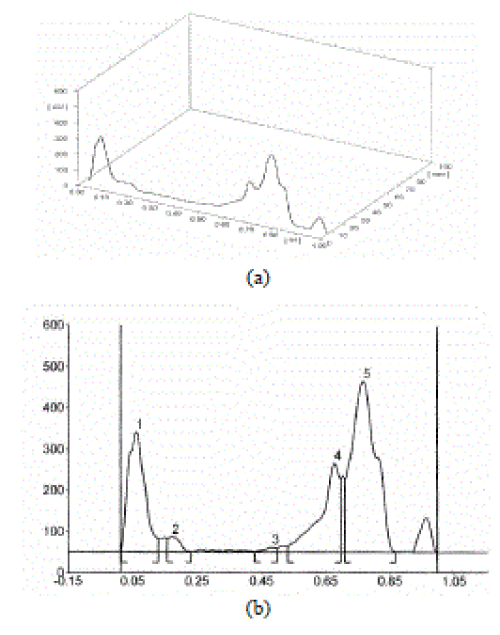
| A densitometric HPTLC analysis was performed to detect the presence of phenolic and antioxidant substances in ethanol extract (Figure 1), as this method provides a rapid detection and localization of the active compounds in a plant extract. The ethanolic extract of aerial parts of M. heterophylla was dissolved with HPLC grade methanol (200 mg/ml). The solution was centrifuged at 3000 rpm for 5 min and used for HPTLC analysis. Then, 2 μl of the samples were loaded as 7 mm band length in the 10 x 10 Silica gel 60F TLC plate using Hamilton syringe and CAMAG Linomat 5 instrument. The samples loaded plate was kept in TLC twin trough developing chamber (after saturation with solvent vapor) and the plate was developed in the respective mobile phase (Toluene-Acetone-Formic acid 4.5:4.5:1) up to 90 mm. The developed plate was dried using hot air to evaporate solvents from the plate and sprayed with stannic chloride reagent. The plate was kept in Photo-documentation chamber (CAMAG REPROSTAR 3) and the images are captured at UV 366 nm. Finally, the plate was fixed in Densitometry TLC Scanner 3 with winCATS software, absorption measurement was done at 254 nm. A similarity was observed in the chromatograms and densitograms. |
| |
| DPPH radical scavenging effect |
| |
| DPPH radical scavenging effects of the extracts were performed according to the method of Blois [12]. Briefly, 0.1 mM solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of extracts solution in water at different concentrations (20, 40, 60, 80, 100 and 150 μg/ml). After 30 min, absorbance was measured at 517 nm. Butylated hydroxyl toluene (BHT) was used as a reference drug. The percentage inhibition was evaluated by comparing the absorbance values of the control and experimental samples. |
| |
| Nitric oxide radical scavenging effect |
| |
| Nitric oxide generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions which were measured by Griess reaction [13-14]. The reaction mixture (3 ml) containing sodium nitroprusside (10 mM) in phosphate buffered saline (PBS) and test extracts in different concentrations (20, 50, 100, 200, 250 and 300 μg/ml) were incubated at 25°C for 150 min. After 30 min, 0.5 ml of the incubated sample was removed and 0.5 ml of Griess reagent (1 % sulphanilamide, 0.1 % naphthylethylene diamine dihydrochloride in 2 % H3PO4) was added. The absorbance of the chromophore formed was measured at 546 nm. Ascorbic acid was used as a standard drug. The percentage inhibition of the nitric oxide radical was evaluated by comparing the absorbance values of the control and experimental samples. |
| |
| Superoxide anion radical scavenging effect |
| |
| Superoxide anion scavenging activities of the extracts were evaluated based on the method described by Nishimiki et al [15] with slight modification. About 1ml of nitroblue tetrazolium (NBT) solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 ml of NADH solution (468 μM in 100 mM phosphate buffer, pH 7.4) and 0.1 ml of sample solution of test extracts (20, 40, 60, 80, 100 and 150 μg/ml) in distilled water were mixed and the reaction started by adding 100 μl of phenazine methosulphate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4). The reaction mixture was incubated at 25°C for 5 min and the absorbance at 560 nm was measured against blank samples. Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. Curcumin was used as reference compound. The percentage of inhibition was determined by comparing the results of control and test samples. |
| |
| Hydroxyl radical scavenging activity |
| |
| The scavenging activity for hydroxyl radicals was measured with Fenton reaction [16]. Reaction mixture contained 60 µl of 1.0mM FeCl3, 90 µl of 1mM 1,10- phenanthroline, 2.4 ml of 0.2 M phosphate buffer (pH 7.8), 150 µl of 0.17 M H2O2, and 1.5 ml of extract at various concentrations. Adding H2O2 started the reaction. After incubation at room temperature for 5 min, the absorbance of the mixture at 560 nm was measured with a spectrophotometer. The hydroxyl radicals scavenging activity was calculated according to the following equation: |
| |
| % Inhibition = ((A0-A1) / A0) × 100) |
| |
| Where A0 was the absorbance of the control (blank, without extract) and A1 was the absorbance in the presence of the extract. |
| |
| Statistical Analysis |
| |
| All data expressed as mean ± SD of three parallel measurements. Data were analyzed by student’s t – test and all results were considered statistically significant at P< 0.05. |
| |
|
RESULTS AND DISSCUSSION
|
| |
| The results of preliminary phytochemical screening of the five extracts revealed the presence of carbohydrates, steroids, flavonoids, saponins, gums and mucilages. Flavonoids are proved to be potent free radical scavenger [17-18]. They are likely to act by scavenging mechanism, sacrificially reduce ROS/ RNS such as ·OH, O2·-, NO· or OONO- after generation, preventing damage to biomolecules or formation of more reactive ROS [19-20]. |
| |
| Total polyphenolics contents of the extracts |
| |
| Phenolic compounds are known as powerful chain breaking antioxidants. The concentration of phenolics in the extract/fraction expressed as µg of pyrocatechol per mg of the sample is shown in Table 1. Ethyl acetate fraction was found as higher phenolics content fraction than others. The high concentration of polyphenolics in the ethyl acetate fraction may be due to purification and concentration of phenolics throughout the fractionation procedure and probably responsible for its high free radical scavenging activity. The FCR reducing capacity of different fractions is due to presence of hydroxyl groups present in the polyphenolics and flavonoids. The key role of phenolic compounds as scavengers for free radicals is emphasized in some reports [21]. They reported that presence of hydroxyl groups contribute directly to antioxidant effect of the system and it also has an important role in stabilizing lipid oxidation. |
| |
|
HPTLC Profile
|
| |
| HPTLC profile of ethanol extract of the aerial parts of M. heterophylla was recorded which confirms the presence of polyphenols (Fig. 1-2). |
| |
| The extracts were run along with the standard polyphenolic compounds. The extract showed the presence of polyphenolic compounds in the chromatograph as well as in UV after derivatization. The Rf value of the different compounds present in the extract was found to be 0.06, 0.17, 0.48, 0.68, 0.77 of peak 1, 2, 3, 4, 5 respectively. Among them peaks 3, 4, 5 were found to contain polyphenolic compounds. The peak height of the respective polyphenols was also given in the Table 2. Melothria heterophylla extracts was found to scavenge DPPH, nitric oxide, superoxide and hydroxyl radicals in vitro in a concentration-dependent manner. |
| |
| DPPH radical scavenging effect |
| |
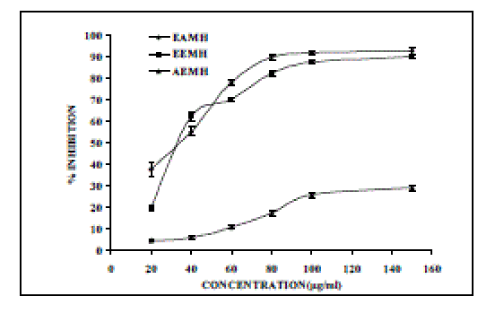
| DPPH radical scavenging activity of different extracts are given in Figure 3. DPPH, a nitrogen centered free radical with a characteristics absorbance at 517 nm and convert to 1,1, diphenyl 2- picryl hydrazine due to its hydrogen accepting ability at a rapid rate.1,1-diphenyl- 2-picrylhydrazyl (DPPH) has been used extensively as a free radical to evaluate reducing substances (22-24). Stable free radicals DPPH were effectively scavenged by M. heterophylla extracts, and the IC50 value of EAMH, EEMH and AEMH were found to be 17.62, 35.17 and 232.91 µg/ml, respectively (Fig. 3). A standard antioxidant BHT was used to compare the antioxidant potential, which exhibited 90.39 % inhibition at a concentration of 100 μg/ml. |
| |
|
Nitric oxide radical scavenging effect
|
| |
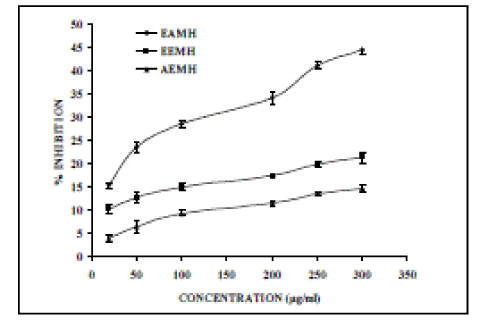
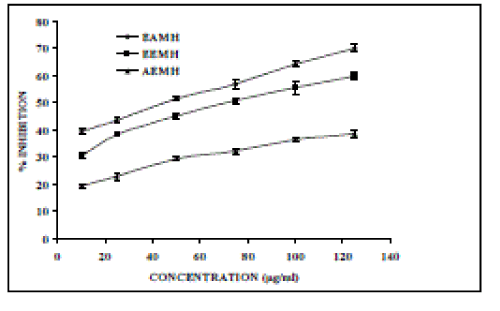
| Nitric oxide (NO) is an important chemical mediator generated by endothelial cells, macrophages, neurons, etc. and involved in the regulation of various physiological processes [25]. Excess concentration of NO is associated with several diseases [26-27]. Oxygen reacts with the excess nitric oxide to generate nitrite and peroxynitrite anions, which act as free radicals [28- 29]. In the present study, the extracts compete with oxygen to react with nitric oxide and thus inhibit generation of the anions. Figure 4, illustrates the percentage inhibition of nitric oxide generation by EAMH, EEMH and AEMH. Ascorbic acid was used as a reference compound, which exhibited 55.88 % inhibition at a concentration of 25 µg/ml. EAMH, EEMH and AEMH shows an IC50 values of 352.8, 1063.37 and 1269.05 µg/ml, respectively. |
| |
| Superoxide anion radical scavenging effect |
| |
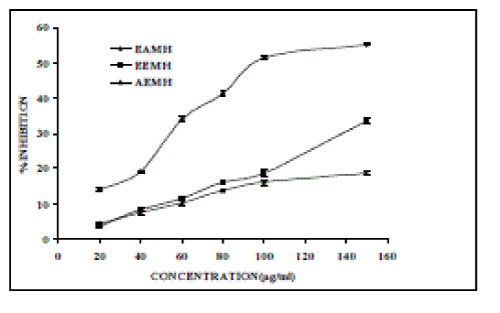
| In the PMS/ NADH-NBT system, superoxide radicals generated from a non enzymatic reaction of PMS in presence NADH and molecular oxygen reduces NBT to formazan at pH 7.8. Figure 5, shows the superoxide scavenging effect of EAMH, EEMH and AEMH on the PMS/NADH-NBT system [30]. |
| |
| The decrease of absorbance at 560 nm with antioxidants thus indicates the consumption of superoxide anion in the reaction mixture. The IC50 values of EAMH, EEMH and AEMH were 116.52, 230.63 and 410.77 µg/ml respectively. Curcumin used as reference, shows 42.21% inhibition at a concentration of 5 µg/ml. |
| |
| Hydroxyl radical scavenging effect |
| |
| Hydroxyl radical is very reactive and can be generated in biological cells through the Fenton reaction. Figure 6, showed the M. heterophylla exhibited concentration dependent scavenging activities against hydroxyl radicals generated in a Fenton reaction system. The concentration of the extracts needed for 50% inhibition of hydroxyl radicals was found to be 47.95, 77.78 and 184.21 µg/ml (Fig. 6). Catechin, used as a standard was highly effective in inhibiting the radicals, showing an IC50 = 5.27 μg/ml. The potential scavenging abilities of phenolic substances might be due to the active hydrogen donor ability of hydroxyl substitution. Similarly, high molecular weight and the proximity of many aromatic rings and hydroxyl groups are more important for the free radical scavenging by specific functional groups [31]. |
| |
CONCLUSION
|
| |
| The results of the current study showed that the extracts of the aerial parts of Melothria heterophylla which contains highest concentration of polyphenols exhibits the greatest antioxidant activity through the scavenging of free radicals such as DPPH radical, nitric oxide, superoxide and hydroxyl radical. So, the extracts of the aerial parts of Melothria heterophylla are the source of natural antioxidants which can be accounted for the traditional uses in prevention of disease and health preservation. |
| |
ACKNOWLEDGEMENTS
|
| |
| The authors are thankful to the University Grant Commission, New Delhi, India for the financial support to the corresponding author and Jadavpur University, Kolkata, for providing research facilities. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
| |












