Keywords
Insulin; Diabetes; Prediabetes; Bacteria; Tuberculosis
Introduction
There is a tremendous increase in incidence of diabetes mellitus throughout the globe and such trend is far from being controlled [1]. Many kinds of infections are associated with diabetes mellitus. So, there is a considerable recent interest in the pathobiology of infections in the diabetic state [2,3]. In type 1 diabetes, insulin production of the host is remarkably low and exogenous supplementation of insulin is necessary to maintain the life process. In prediabetes and type 2 diabetes hyperinsulinemia is documented [4,5]. In advanced type 2 diabetes insulin production tends to decline [6]. However, irrespective of insulin level, infections are reported to be more in diabetes mellitus [7].
Type 2 Diabetes mellitus is a recognized risk factor for tuberculosis and other infections [8]. Such is the case for prediabetes and type 1 diabetes too [9,10]. Conditions associated with prediabetes like obesity are also known to exhibit increased rate of infections [8].
With this background information, we feel that effect of insulin per se on bacterial growth needs investigation. Data generated from such research has the potential to provide novel insights in the pathobiology of infections in the diabetic state. It is believed by the scientific community that the pathogenesis of infections in the diabetic state is multi-factorial [8]. However, the direct effect of insulin on bacterial growth is not investigated so far. Keeping in mind, the increasing trend of diabetes throughout the globe we feel that this issue warrants investigation.
Materials and Methods
Bacterial strains
The following strains were obtained for the purpose of this study:
1. Mycobacterium tuberculosis H37Ra
2. Mycobacterium tuberculosis H37Rv
3. Mycobacterium bovis BCG
4. Escherichia coli (MTCC 9492)
5. Staphylococcus aureus (MTCC 3160)
Bacteriological media
The following bacteriological media were obtained from Himedia Laboratories, Mumbai, India:
1. L.J. Medium (LOT SL0010)
2. Middlebrook 7H10 Agar Base (LOT0000168949)
3. Modified Middlebrook 7H9 Broth Base with Indicator (LOT MTO001)
4. Nutrient Agar (LOT0000134112)
5. Nutrient Broth No.3 (LOT0000179752)
6. Muller Hinton Agar (LOT0000121686)
Insulin
Human recombinant insulin (Wosulin, Wockhardt Ltd.) containing 40 IU/ml regular insulin with 0.25% m-cresol w/v was used for this study. It was diluted with appropriate bacteriological media to achieve the studied concentrations. m-cresol (Thomas Baker, Minimum Assay (GC) 99%) was also diluted in appropriate media for the purpose of control.
Insulin concentrations used were 5 μIU/ml (0.035 nM), 10 μIU/ml (0.07 nM), 100 μIU/ml (0.7 nM), 200 μIU/ml (1.4 nM), 400 μIU/ml (2.8 nM), 700 μIU/ml (4.9 nM), 1000 μIU/ml (6.9 nM), 0.5 IU/ml (3472.5 nM), 1 IU/ml (6945 nM), 2 IU/ml (13890 nM), 4 IU/ml (27780 nM), 10 IU/ml (69450), 20 IU/ml (138900 nM), 30 IU/ml (208350 nM), 40 IU/ml (277800 nM).
Of the above mentioned insulin concentration 5 μIU/ml (0.035 nM), 10 μIU/ml (0.07 nM) are within physiological concentration because 2-25 μIU/ml is the known physiological range of concentration for Insulin in normal individuals [11].
Determining the effect of insulin on mycobacterial growth
1 McFarland standard suspensions of above mentioned mycobacterial strains were prepared. 10 μL from each mycobacterial suspensions (1 McFarland std.) were added to 1 ml of Middlebrook 7H9 broth containing different insulin concentrations in separate aliquots. The chosen insulin concentrations were 5 μIU/ml, 10 μIU/ml, 100 μIU/ml, 0.5 IU/ml, 1 IU/ml, 2 IU/ml, 4 IU/ml, 10 IU/ml, 20 IU/ml, 30 IU/ml, and 40 IU/ml. Equal amount of mycobacterial suspension were added in Middlebrook 7H9 containing the corresponding amount of m-cresol for the purpose of control. Both sets of inoculum were kept for incubation at 37°C under the sterile conditions. After an incubation of 18 hours, 100 μL from each aliquot was spread on the surface of LJ medium slants and kept for incubation at 37°C under sterile condition and checked for visible growth every week up to eight weeks.
Agar well diffusion with other bacterial strains
0.5 McFarland standard suspensions of Escherichia coli MTCC 9492, Staphylococcus aureus MTCC 3160 were prepared in sterile Nutrient Broth and Muller Hinton Broth. Lawn cultures of the above mentioned bacterial strains were drawn up on the solid surface of Nutrient Agar and Muller Hinton Agar media from their corresponding 0.5 McFarland standard suspensions (mentioned above) by using sterile cotton swabs. Wells were cut on the surface of agar media in such a way so that it can hold a 400 μL volume of 400 IU insulin or corresponding controls in the separate experimental system. Well diffusion was performed to check insulin effect on bacterial growth using 400 μL of insulin and incubated overnight before checking for a Zone of Inhibition (ZoI).
Study of insulin effect on growth by broth dilution
A set of 9 aliquots were taken. First two aliquots were containing 1 ml sterile Nutrient Broth without insulin. The rest seven were holding 1 ml Nutrient broth medium with different concentrations of insulin. The concentrations chosen were 1 IU/ml, 2 IU/ml, 4 IU/ml, 10 IU/ml, 20 IU/ml, 30 IU/ml, and 40 IU/ml of insulin respectively. All these concentrations of insulin contained m-cresol since it is diluted from a formulation that contains m-cresol as preservative. So, a similar set of aliquots were taken as corresponding controls that does not contain insulin but contains equivalent amount of m-cresol. The corresponding controls are prepared for each insulin concentration that contain equivalent amount of m-cresol. For 1 IU/ml, 2 IU/ml, 4 IU/ml, 10 IU/ml, 20 IU/ml, 30 IU/ml, and 40 IU/ml of insulin the corresponding controls were 62.5 μg/ml m-cresol, 125 μg/ml m-cresol, 250 μg/ml m-cresol, 625 μg/ml m-cresol, 1250 μg/ml m-cresol, 1875 μg/ml m-cresol, and 2500 μg/ml m-cresol respectively. 10 μL of 0.5 McFarland standard suspension of inoculums (Escherichia coli MTCC 9492 or Staphylococcus aureus MTCC3160) were added to the abovementioned aliquots. All aliquots were kept for incubation at 37°C for 18 hours in a shaker incubator. After incubation 10 μL from each of the aliquots were spot inoculated on the Nutrient agar surface. It was once again incubated for 18 hours and checked for growth.
Photometric assessment of bacterial growth
A set of 5 aliquots containing nutrient broth inoculated with bacteria containing different concentrations of insulin were taken. The chosen concentrations of insulin were 0.5 IU/ml, 1 IU/ml, 2 IU/ ml, 4 IU/ml and 10 IU/ml insulin. Separate set of 5 aliquots with corresponding m cresol control were inoculated with Escherichia coli MTCC 9492. Since the insulin formulation used in the study contains m-cresol separate sets of controls with equivalent amount of m-cresol was considered necessary to interpret the results. Both the test and controls were kept in a shaker incubator at 37°C under sterile condition up to 48 hours. Absorbance at 540 nm was noted after every two hours interval from the inoculated media till 12 hours and then absorbance was noted at 24th, 36th and 48th hours. Similar experiments were done with Staphylococcus aureus MTCC3160. With Staphylococcus aureus, same experiments were performed in nutrient broth added with 0.2% glucose. With Escherichia coli (MTCC 9492) similar sets of experiments were repeated with different concentrations of insulin. The concentrations chosen were 10 μIU/ml, 100 μIU/ml, 200 μIU/ml, 700 μIU/ml, and 1000 μIU/ml of insulin. Appropriate controls were taken in different aliquots. For 10 μIU/ml, 100 μIU/ml, 200 μIU/ ml, 700 μIU/ml, and 1000 μIU/ml of insulin the appropriate controls were 0.000625 μg/ml m-cresol, 0.0062 5 μg/ml m-cresol, 0. 0125 μg/ ml m-cresol, 0.0175 μg/ml m-cresol and 0.0625 μg/ml m-cresol respectively. The experiments were continued up to the 8th hour. The Blank for the photometric experiment was sterile Nutrient Broth.
Colony count
Escherichia coli: 100 μl of 0.5 McFarland bacteria was added to 10 ml nutrient broth containing 0.5 IU/ml insulin, 1 IU/ml Insulin and 2 IU/ml insulin and incubated at 37°C for 18 hours in a shaker incubator. After the incubation a serial dilution (102 to 109) were made in Nutrient Broth, and 10 μl from 104 and 106 dilution of E. Coli were spread uniformly on the sterile agar surface by using a sterile spreader (L-rod). This process was continued at an interval of every two hours. After an incubation period of 18 hours, colony count was performed. In separate aliquot same experiment was repeated with an appropriate control containing equivalent amount of m-cresol.
Staphylococcus aureus: A similar pattern of inoculation with 100 μl of 0.5 McFarland bacteria with 1 IU/ml insulin resulted in very less colony (<30 cfu). So, here 200 μl of 0.5 McFarland of bacterial growth was added to 10 ml nutrient broth containing 0.5 IU/ml Insulin, 1 IU/ ml insulin and 2 IU/ml insulin and incubated at 37°C for 18 hours in a shaker incubator. After the incubation, a serial dilution was made, and 10 μl from 109 dilution was spread uniformly on the sterile agar surface by using a sterile spreader (L-rod). After an incubation period of 18 hours colony count was performed. In separate aliquot, same experiment was repeated with an appropriate control containing an equivalent amount of m-cresol.
Results
With mycobacterial species
All the positive controls (bacterial strain without insulin or cresol) have shown growth of Mycobacterium tuberculosis H37Ra, Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG respectively as expected.
Colonies of Mycobacterium tuberculosis H37Ra repeatedly appeared after 4-5 weeks on the surface of LJ medium slants where the inoculums were incubated for 18 hours with 4 IU/ml, 2 IU/ml, 1 IU/ml, and 0.5 IU/ml of insulin. However, there was no trace of mycobacterial growth on the Lowenstein-Jensen (LJ) medium slants where the inoculums were incubated with corresponding m-cresol controls.
No growth on LJ medium slants were observed where inoculums were incubated with higher concentrations of insulin (10 IU/ml, 20 IU/ ml, 30 IU/ml, and 40 IU/ml). In the corresponding m-cresol control, growth was also not observed. In the case of inoculation with 5 μIU/ ml, 10 μIU/ml and 100 μIU/ml insulin growth was observed on LJ slant. However in the corresponding control similar growth was also observed. Similar results were observed with other mycobacterial species chosen for experimentation (Figure 1). All experiments were repeated thrice and similar results yielded.
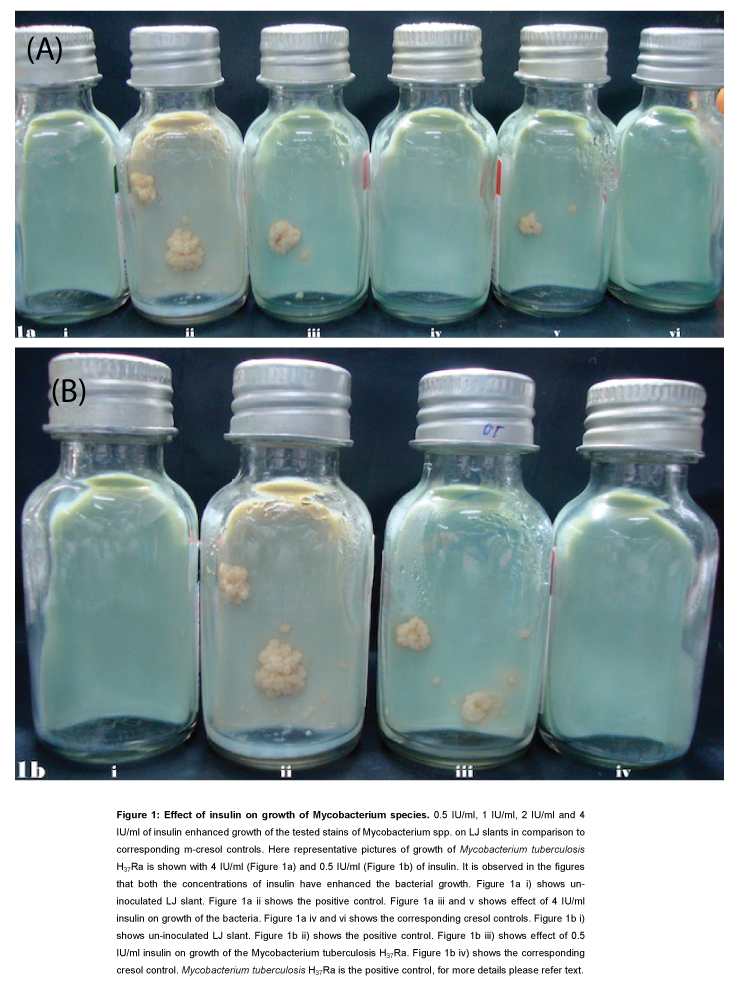
Figure 1: Effect of insulin on growth of Mycobacterium species. 0.5 IU/ ml, 1 IU/ml, 2 IU/ml and 4 IU/ml of insulin enhanced growth of the tested stains of Mycobacterium spp. on LJ slants in comparison to corresponding m-cresol controls. Here representative pictures of growth of Mycobacterium tuberculosis H37Ra is shown with 4 IU/ml (Figure 1a) and 0.5 IU/ml (Figure 1b) of insulin. It is observed in the figures that both the concentrations of insulin have enhanced the bacterial growth. Figure 1a i) shows un-inoculated LJ slant. Figure 1a ii shows the positive control. Figure 1a iii and v shows effect of 4 IU/ml insulin on growth of the bacteria. Figure 1a iv and vi shows the corresponding cresol controls. Figure 1b i) shows un-inoculated LJ slant. Figure 1b ii) shows the positive control. Figure 1b iii) shows effect of 0.5 IU/ ml insulin on growth of the Mycobacterium tuberculosis H37Ra. Figure 1b iv) shows the corresponding cresol control. Mycobacterium tuberculosis H37Ra is the positive control, for more details please refer text.
With other bacterial species
The chosen strains of Mycobacterium spp. are very slow growing. So, further confirmation of the growth enhancement effect was done with other bacteria. Experiments were designed to determine the effect of insulin on the growth of Escherichia coli MTCC 9492 and Staphylococcus aureus MTCC 3160.
Agar well diffusion
Escherichia coli: The Zone of Inhibition (ZoI) with 400 μL Insulin preparation containing m-cresol was found to be lower than the zone of inhibition with corresponding m-cresol control (Table 1 and Figure 2a). It indicates that inhibitory effect of m-cresol is reduced by the insulin preparation used that contains equivalent amount of m-cresol.
| |
Nutrient Agar |
Muller Hinton Agar |
Insulin with
0.25% m-cresol |
Corresponding
m-cresol control |
Insulin with
0.25% m-cresol |
Corresponding
m-cresol control |
| Escherichia coli |
30 mm |
34 mm |
33 mm |
38 mm |
| 32 mm |
36 mm |
32 mm |
38 mm |
| 30 mm |
36 mm |
32 mm |
36 mm |
| Staphylococcus aureus |
14 mm |
18 mm |
20 mm |
24 mm |
| 12 mm |
18 mm |
18 mm |
22 mm |
| 14 mm |
18 mm |
20 mm |
26 mm |
Table 1: Agar well diffusion. A prototype result of measurement of diameter of the zones of Inhibition (ZoI) around the wells holding 400 μL of human recombinant insulin and corresponding m-cresol control on Nutrient Agar and Muller Hinton Agar. Bacterial strains used are Escherichia coli MTCC 9492, Staphylococcus aureus MTCC 3160. Please refer corresponding Figure 2.
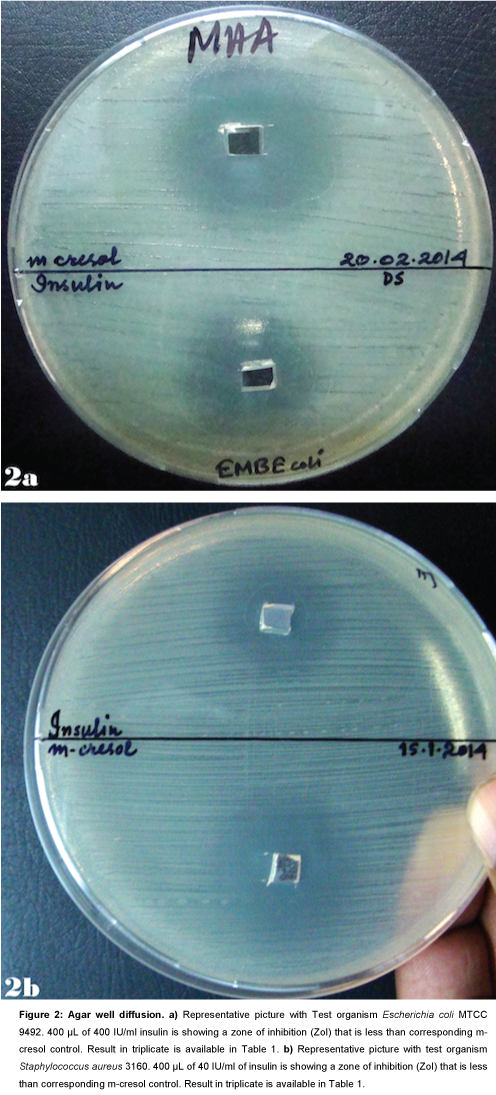
Figure 2: Agar well diffusion. a) Representative picture with Test organism Escherichia coli MTCC 9492. 400 μL of 400 IU/ml insulin is showing a zone of inhibition (ZoI) that is less than corresponding m- cresol control. Result in triplicate is available in Table 1. b) Representative picture with test organism Staphylococcus aureus 3160. 400 μL of 40 IU/ml of insulin is showing a zone of inhibition (ZoI) that is less than corresponding m-cresol control. Result in triplicate is available in Table 1.
Staphylococcus aureus: The Zone of Inhibition (ZoI) with 400 μL Insulin preparation containing m-cresol was found to be lower than the zone of inhibition with corresponding m-cresol control (Table 1 and Figure 2b). It indicates that inhibitory effect of m-cresol is reduced by the insulin preparation used that contains equivalent amount of m-cresol.
Studying insulin effect on growth by broth dilution
When the growth from equal dilutions of insulin formulation with m-cresol and corresponding m-cresol control were compared, insulin preparation was found to support the bacterial growth in liquid media.
Up to 10 IU/ml insulin formulation and corresponding m-cresol control in Nutrient broth supported the growth of Escherichia coli MTCC 9492. 20 IU/ml insulin formulation supported the bacterial growth, but corresponding m-cresol control completely inhibited the growth. 30 IU/ml and 40 IU/ml and also its corresponding m-cresol controls respectively inhibited the growth of E. coli MTCC 9492 (Table 2 and Figure 3a).

Figure 3: Assaying insulin effect on growth by broth dilution. Effect on Escherichia coli growth. 1 IU (i2), 2 IU (i3), 4 IU (i4), and 10 IU (i5) of insulin and corresponding m-cresol controls (CR1, CR3, CR4, CR5) supporting growth of Escherichia coli MTCC 9492. 20 IU (i6) insulin supports the bacterial growth but corresponding m-cresol control CR6 shows complete inhibition. 30 IU (i7) and 40 IU (i8) insulin as well as their corresponding m-cresol controls (CR7, CR8) showing complete inhibition of growth. SL1-8 and DW1-8 are corresponding dilutions with sterile normal saline and distilled water to nullify the concept that such dilution method may affect bacterial growth.
Effect of insulin on the growth of Staphylococcus aureus MTCC 3160 was observed with concentrations in ascending order in a similar manner. Here, the corresponding concentration of m-cresol present in 2 IU/ml insulin showed growth inhibition, but 2 IU/ml insulin formulation showed confluent growth even in the presence of the same concentration of m-cresol as a preservative. 4 IU/ml, 10 IU/ ml, 20 IU/ml insulin also observed to support the growth where the corresponding m-cresol controls showed marked inhibition. 40 IU Insulin and corresponding m-cresol control had exhibited complete inhibition (Table 2 and Figure 3b).
| Escherichia coli |
| Insulin |
Growth |
m-cresol |
Growth |
Saline |
Growth |
D. water |
Growth |
Sterile Nutrient broth
(Neg. control) |
No growth (-) |
Sterile Nutrient broth (Neg. control) |
No growth (-) |
Sterile Nutrient broth (Neg. control) |
No growth (-) |
Sterile Nutrient broth (Neg. control) |
No growth (-) |
| 0 IU/ml (Pos. control) |
+ (Confluent) |
No m-cresol |
+ (Confluent) |
No saline added |
+ (Confluent) |
No distilled water added |
+ (Confluent) |
| 1 IU/ml |
+ (Confluent) |
Corresponding m-cresol |
+ (Confluent) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 2 IU/ml |
+ (Confluent) |
Corresponding m-cresol |
+ (Confluent) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 4 IU/ml |
+ (Confluent) |
Corresponding m-cresol |
+ (less than 2IU) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 10 IU/ml |
+ (less than 4IU) |
Corresponding m-cresol |
+(very few) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 20 IU/ml |
+ (very few) |
Corresponding m-cresol |
No growth (-) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 30 IU/ml |
No growth (-) |
Corresponding m-cresol |
No growth (-) |
Corresponding vol. of saline |
+ (Confluent) |
Corresponding vol. D. water |
+ (Confluent) |
| 40 IU/ml |
No growth (-) |
Corresponding m-cresol |
No growth (-) |
Sterile normal saline |
+ (Few) |
Sterile D. sterile |
+ (Few) |
Table 2: Study of effect of insulin on bacterial growth (Escherichia coli MTCC 9492). 4 IU/ml, 10 IU/ml, 20 IU/ml concentrations of insulin showing possible support to bacterial growth, but corresponding cresol controls showing marked inhibition. Please refer corresponding Figure 3.
Photometric study of bacterial growth with Escherichia coli and Staphylococcus aureus
Escherichia coli: Increasing absorbance values at 540 nm were observed with 0.5 IU/ml, 1 IU/ml, 2 IU/ml insulin than normal positive control (sterile nutrient broth inoculated with 0.5 McFarland unit) and corresponding m-cresol controls (Figure 4a). In higher concentrations of insulin formulation, initial inhibition was observed where m-cresol concentrations were high. Initially inhibited bacterial growth (with lower absorbance values) shown to be stimulated on prolonged incubation with higher concentrations of insulin. The growth enhancement by insulin was observed in both presence and absence of glucose (in nutrient broth with and without added glucose 0.2%).
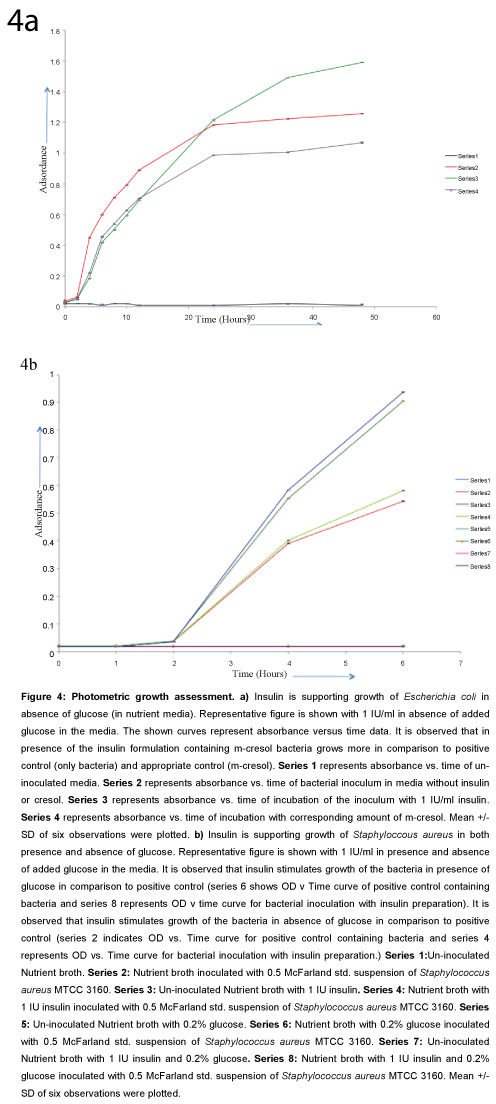
Figure 4: Photometric growth assessment. a) Insulin is supporting growth of Escherichia coli in absence of glucose (in nutrient media). Representative figure is shown with 1 IU/ml in absence of added glucose in the media. The shown curves represent absorbance versus time data. It is observed that in presence of the insulin formulation containing m-cresol bacteria grows more in comparison to positive control (only bacteria) and appropriate control (m-cresol). Series 1 represents absorbance vs. time of un-inoculated media. Series 2 represents absorbance vs. time of bacterial inoculum in media without insulin or cresol. Series 3 represents absorbance vs. time of incubation of the inoculum with 1 IU/ml insulin. Series 4 represents absorbance vs. time of incubation with corresponding amount of m-cresol. Mean +/- SD of six observations were plotted. b) Insulin is supporting growth of Staphyloccous aureus in both presence and absence of glucose. Representative figure is shown with 1 IU/ml in presence and absence of added glucose in the media. It is observed that insulin stimulates growth of the bacteria in presence of glucose in comparison to positive control (series 6 shows OD v Time curve of positive control containing bacteria and series 8 represents OD v time curve for bacterial inoculation with insulin preparation). It is observed that insulin stimulates growth of the bacteria in absence of glucose in comparison to positive control (series 2 indicates OD vs. Time curve for positive control containing bacteria and series 4 represents OD vs. Time curve for bacterial inoculation with insulin preparation.) Series 1:Un-inoculated Nutrient broth. Series 2: Nutrient broth inoculated with 0.5 McFarland std. suspension of Staphylococcus aureus MTCC 3160. Series 3: Un-inoculated Nutrient broth with 1 IU insulin. Series 4: Nutrient broth with 1 IU insulin inoculated with 0.5 McFarland std. suspension of Staphylococcus aureus MTCC 3160. Series 5: Un-inoculated Nutrient broth with 0.2% glucose. Series 6: Nutrient broth with 0.2% glucose inoculated with 0.5 McFarland std. suspension of Staphylococcus aureus MTCC 3160. Series 7: Un-inoculated Nutrient broth with 1 IU insulin and 0.2% glucose. Series 8: Nutrient broth with 1 IU insulin and 0.2% glucose inoculated with 0.5 McFarland std. suspension of Staphylococcus aureus MTCC 3160. Mean +/- SD of six observations were plotted.
With Escherichia coli it was observed that 1000 micro IU/ml insulin is causing 4.5% growth enhancement in 8th hour (OD in positive control in 8th hour 1.11392 +/- 0.00107, OD with 1000 micro IU/ml insulin in 8th hour 1.16067+/- 0.00193; n=6).
Staphylococcus aureus: With 1 IU/ml and 2 IU/ml of insulin growth enhancement effect is observed with Staphylococcus aureus in presence and absence of glucose. With lower concentration of insulin (<0.5 IU/ml) growth enhancement effect was not observed (Figure 4b).
Colony count
Colony count had shown higher counts with 0.5 IU, 1 IU/ml, 2 IU/ml insulin than normal positive control (sterile nutrient broth inoculated with 0.5 McFarland unit) in Nutrient Agar. It was observed with and without added glucose.
For E. coli with 2 IU/mL insulin preparation it is observed that in presence of insulin 12 fold more colony counts are increased in comparison to appropriate control (Figure 5a).
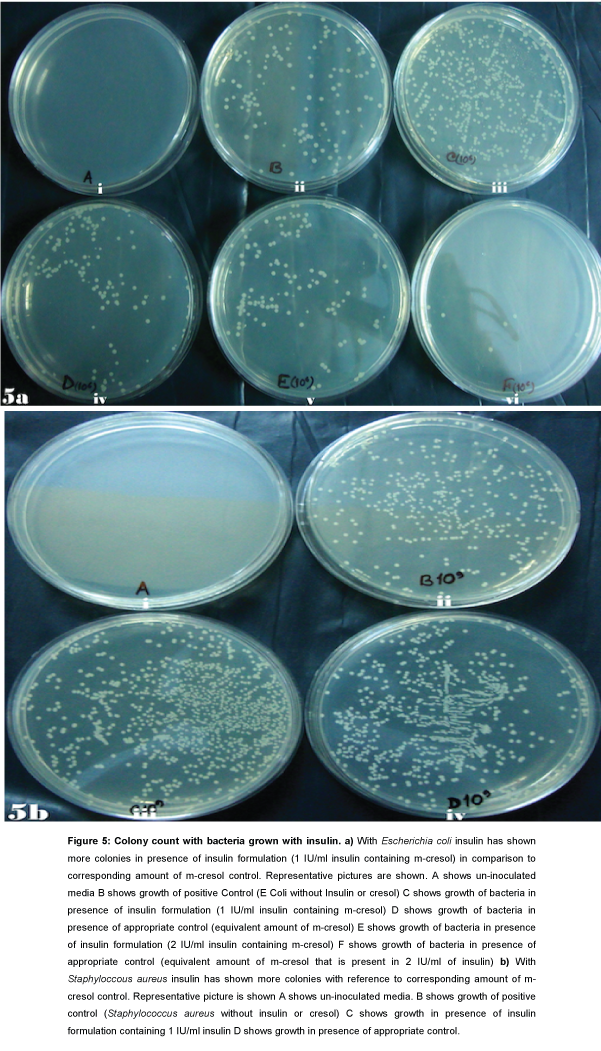
Figure 5: Colony count with bacteria grown with insulin. a) With Escherichia coli insulin has shown more colonies in presence of insulin formulation (1 IU/ml insulin containing m-cresol) in comparison to corresponding amount of m-cresol control. Representative pictures are shown. A shows un-inoculated media B shows growth of positive Control (E Coli without Insulin or cresol) C shows growth of bacteria in presence of insulin formulation (1 IU/ml insulin containing m-cresol) D shows growth of bacteria in presence of appropriate control (equivalent amount of m-cresol) E shows growth of bacteria in presence of insulin formulation (2 IU/ml insulin containing m-cresol) F shows growth of bacteria in presence of appropriate control (equivalent amount of m-cresol that is present in 2 IU/ml of insulin) b) With Staphyloccous aureus insulin has shown more colonies with reference to corresponding amount of m-cresol control. Representative picture is shown A shows un-inoculated media. B shows growth of positive control (Staphylococcus aureus without insulin or cresol) C shows growth in presence of insulin formulation containing 1 IU/ml insulin D shows growth in presence of appropriate control.
For Staphylococcus aureus with 1 IU/ml insulin preparation 1.7 fold increases of colony count is observed in comparison to appropriate control (Figure 5b).
Discussion
Infections are observed to be more in diabetes mellitus [2,3]. It is observed to be more in type 1 diabetes where insulin is produced less in the body. Infections are also frequent in prediabetes and type 2 diabetes mellitus where hyperinsulinemia is documented. It is in this context our observations are relevant.
In the present study, it is observed that the tested physiological concentrations [11] of the concerned formulation of insulin does not support or inhibit the growth of the strains of bacteria. So, it appears that physiological concentrations of insulin may not have any effect on bacterial growth. The high concentrations of insulin (100 μIU/ml, 200 μIU/ml, 400 μIU/ml and 700 μIU/ml) that are tested also do not elicit any effect on bacterial growth. With 1000 μIU/ml of insulin growth of Escherichia coli is observed to be supported. However, very high doses of insulin (0.5 IU/ml, 1 IU/ml, 2 IU/ml, and 4 IU/ml) supported the growth of the tested strains of bacteria. Such effect on bacterial growth appears to be independent of glucose since in nutrient agar/ broth where glucose is not an ingredient has shown significant bacterial growth enhancement in the presence of higher doses of insulin. In an earlier study, it has been shown that insulin supports growth of bacteria in a glucose dependent manner [12]. However, to the best of our knowledge glucose-independent bacterial growth enhancement by supra-physiological doses of human recombinant insulin is reported for the first time. It is observed in this study that 40 IU/ml of insulin preparation inhibits growth of E. coli. This does not appear to be the effect of insulin because higher dose of insulin from the test formulation also contained higher amount of m-cresol which is expected to show growth inhibition with higher dose of insulin formulation.
It is a fact that portal circulation has more insulin concentration than the systemic circulation [13]. It is further known that portal circulation insulin concentration rises significantly particularly after glucose challenge [14]. So, in situations like prediabetes, where insulin in the portal circulation increases significantly [14], can itself be an independent risk factor for infections in liver. Interestingly, in diabetes mellitus pyogenic liver abscess (PLA) caused by Escherichia coli is reported significantly more [15]. Therefore, the higher insulin concentration of portal circulation in prediabetes or type 2 diabetes can be a causative factor for increased association of PLA in diabetic state.
Further in animal model of type 2 diabetes mellitus infected with tuberculosis (Mycobacterium tuberculosis H37Rv) more bacterial burden was observed in the liver homogenate on the 90th day post infection [16]. Our study documents that higher concentration of human recombinant insulin supports growth of H37Rv strain of Mycobacterium tuberculosis. Therefore, it is possible that higher portal concentration of insulin in impaired glucose tolerance can aid to the proliferation of pathogenic strain of Mycobacterium tuberculosis in hepatobiliary system. In this context, it is interesting to note that the incidence of military tuberculosis particularly with pulmonary involvement is more in diabetes with poor glycemic control [17]. So, increased portal concentration of insulin in impaired glucose tolerance has the potential to precipitate tuberculosis infection in liver if there is spread from lungs.
Other than pathological consequences of higher concentration of portal insulin in diabetes our study has relevance in novel antidiabetic drug development for the management of diabetes mellitus. One of the current approaches of management of diabetes mellitus is to reprogram the intestinal cells to secrete insulin [18]. If such approach becomes a reality, then there will be a local delivery of high concentration of insulin in the gastrointestinal system. In such case, it is possible that local high concentration of insulin may exhibit growth promoting effect on the available microbiome. Therefore, we strongly feel that the effect of higher doses of insulin on bacterial growth is an important phenomenon with relevance to development of novel antidiabetic strategies as well as pathobiology of infections in diabetic state.
Acknowledgements
This work is an outcome of working out a project proposal entitled “Evaluation of human recombinant and other forms of insulin for reduction of Mycobacterium tuberculosis proliferation in in vitro cell culture system - a step towards novel antitubercular drug discovery (BT/PR3236/MED/30/644/2011)” funded by Department of Biotechnology, Government of India. DB and SC acknowledge the funding agency for financial assistance.
9932
References
- Noemí Santos-Caballero, Francisco Javier Flores-Murrieta, Comparative Pharmacokinetic Study between Metformin Alone and Combined with Orlistat in Healthy Mexican Volunteers
- Casqueiro J,Casqueiro J, Alves C (2012) Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J EndocrinolMetab 16 Suppl 1: S27-36.
- Nitzan O, Elias M, Chazan B, Saliba W (2015) Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes MetabSyndrObes 8: 129-136.
- Saul M. Genuth, MD,1 Jerry P. Palmer, MD,2 and David M. Nathan, MD3.
, Classification and Diagnosis of Diabetes
- Li C, Ford ES, Zhao G, Mokdad AH (2009) Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care 32: 342-347.
- Clark A, Jones LC, de Koning E, Hansen BC, Matthews DR (2001) Decreased insulin secretion in type 2 diabetes: a problem of cellular mass or function? Diabetes 50 Suppl 1: S169-171.
- Muller LM,Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, et al. (2005) Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 41: 281-288.
- Huttunen R,Syrjänen J (2013) Obesity and the risk and outcome of infection. Int J Obes (Lond) 37: 333-340.
- Viswanathan V,Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, et al. (2012) Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One 7: e41367.
- Simonsen JR,Harjutsalo V, Järvinen A, Kirveskari J, Forsblom C, et al. (2015) Bacterial infections in patients with type 1 diabetes: a 14-year follow-up study. BMJ Open Diabetes Res Care 3: e000067.
- Sacks DB (1999) Carbohydrates. In: Burtis CA, Ashwood ER,Tietz NW (Eds)TietzTextbook of Clinical Chemistry. WB Saunders Company,Philadelphia,USA, pp: 750-808.
- Plotkin BJ,Viselli SM (2000) Effect of insulin on microbial growth. CurrMicrobiol 41: 60-64.
- Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, et al. (2000) Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J ClinEndocrinolMetab 85: 4491-4499.
- R M Walter Jr, E M Gold, C A Michas, J W Ensinck
, Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion
- Chen SC, Yen CH, Lai KC, Tsao SM, Cheng KS, et al. (2005) Pyogenic liver abscesses with Escherichia coli: etiology, clinical course, outcome, and prognostic factors. Wien KlinWochenschr 117: 809-815.
- Podell BK, Ackart DF, Obregon-Henao A, Eck SP, Henao-Tamayo M, et al.(2014) Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: a model of diabetes-tuberculosis comorbidity. Am J Pathol 184:1104-1118.
- Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, et al. (2008) Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 167: 1486-1494.
- Duan FF, Liu JH, March JC (2015) Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 64: 1794-1803.











