Keywords
Dissemination; Strongyloidiasis; Immunocompromised
Introduction
Strongyloidiasis is an intestinal infection caused by Strongyloides stercoralis, an intestinal nematode, which infects millions of people worldwide [1]. It is common in the tropical, subtropical and temperate regions. It is endemic in Southeast Asia, Latin America, and Sub-Saharan Africa and in the South Eastern United States. The infection is usually asymptomatic in the immunocompetent host with mild gastrointestinal symptoms, but involves multiple organs in immunocompromised individuals.
Strongyloidiasis can be clinically inapparent or manifests as cutaneous symptoms (serpiginous rash termed larva currens), or mild gastrointestinal symptoms (abdominal discomfort, diarrhea, nausea, loss of appetite) or pulmonary symptoms (cough and breathlessness) [2]. We report a case of hyperinfection with S. stercoralis in an immunocompromised patient.
Case Report
A 48 years old renal transplant patient who was on immunosuppressive therapy with triple regime (tacrolimus 4 mg, Mycophenolatemofetil 1.5 g, Wysolone 20 mg), developed abdominal pain following intake of food or water . He also complained of dysuria after 3 months of surgery. Clinically the patient was stable.
Laboratory investigations revealed eosinophil count of 10% at the time of admission with total count of 18,000 (Table 1).Ultra sonogram of abdomen showed calcified granuloma in spleen, suggestive of post infectious etiology. Contrast Enhanced Computed Tomography (CECT) abdomen was suggestive of subtle dilatation of small bowel loops including duodenum and show feces in lumen. Upper gastrointestinal endoscopy (UGIE) showed small pin head sized white patch at D2. Biopsy taken from D2 and esophagus was suggestive of Strongyloidiasis. Wet film of stool showed larva of S. stercoralis.
| Parameters |
Day1 |
Day 3 |
Day5 |
Day 7 |
Day 9 |
| Total count |
18,000 |
14,000 |
11,600 |
8400 |
8200 |
| Eosinophil% |
10 |
6 |
2 |
2 |
1 |
Table 1: Blood counts of the patient during the hospital admission.
Patient was started on oral tablet of ivermectin 20mg/kg but he developed shortness of breath with bilateral crepitations. Diffuse alveolar hemorrhage was seen on Fibreoptic bronchoscopy and wet film of bronchoalveolar lavage (BAL) showed live and motile larva of S. Stercoralis (Figure 1). Aerobic culture of the BAL showed growth of Klebsiellapneumoniae, which was sensitive only to colistin for which he was treated with Inj. colistin 1 mU twice daily. Patient was continued on ivermectin and albendazole 400 mg twice daily. Patient’s general condition became stable. On the patient’s request, he was discharged with an advice to continue Ivermectin and albendazole for 2 weeks. On follow up, the patient’s stool examination was negative for S. stercoralis.
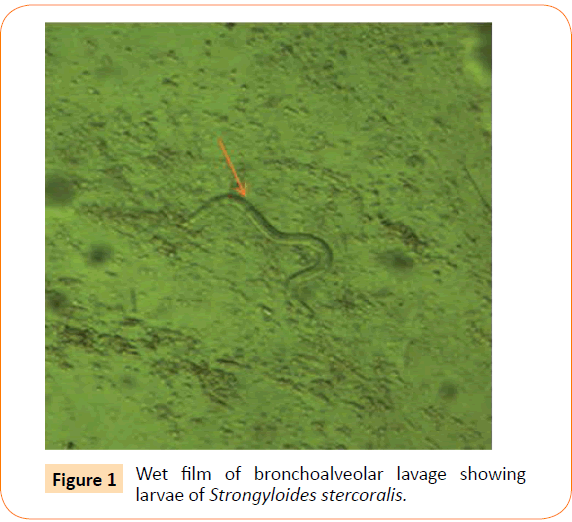
Figure 1:Wet film of bronchoalveolar lavage showing larvae of Strongyloides stercoralis.
Discussion
Infection with S. stercoralis was first reported in the year 1876 in French soldiers working in Vietnam, however, the clinical features of Strongyloidiasis were first described by Fulleborn in 1926 [1,3]. The first report of disseminated infection or hyperinfection dates back to 1966, when Cruz et al., and Rogers et al., independently documented the occurrence of fatal strongyloidiasis with immunosuppression [3].
In India, the prevalence of strongyloidiasis in community based survey is 6.6% and hospital based survey is 11.2% [4]. Studies from various parts of Andhra Pradesh have shown that the prevalence of S. stercoralis, in diarrhea patients, ranges from 0.39-2% in the study regions [5-7]. Earlier, two cases of hyperinfection in immunocompromised patients were recorded (unpublished data) at our Institute.
S. stercoralis is unique among common helminthes for its potential for autoinfection and persistence and is more frequently found in immunosuppressed individuals, socio economically disadvantaged and persons walking on bare feet. Dissemination or hyperinfection occurs in patients with human T-cell lymphotropic virus type-1 infections (HTLV-1), hematologic malignancies, or others receiving corticosteroids, chemotherapy, or immunosuppressive for organ transplantation [2]. In immunosuppressed patients autoinfection leads to overwhelming number of migrating larva leading to dissemination which involves multiple organs or hyperinfection limited to gastrointestinal and respiratory tract as in our case [2,4].
In hyperinfection or dissemination, the worms, while migrating across the intestinal mucosa carry the enteric flora into the blood stream leading to Gram negative bacteremia [2].
Immunocompromised states associated with HIV and HTLV-1, are predominantly due tothe infection of the T cells resulting in decreased production of IL-4, IL-5, IL-13 and IgE, molecules that participate in the host defense mechanism against helminthes. Moreover, there is a decreased response to treatment for S. stercoralis in these patients, which is the most important risk factor for disseminated strongyloidiasis [8].
The diagnosis of strongyloidiasis should be suspected if there are clinical signs and symptoms related to the infection, either GI or respiratory, along with eosinophilia. However, eosinophilia is absent in disseminated disease, indicating a poor prognosis [9]. Our patient had an initial eosinophilia, which became normal after treatment.
The gold standard for diagnosis of strongyloidiasis is detection of larvae in the clinical specimen, such as feces, bronchial wash and sputum. Other methods of diagnosis include stool concentration techniques, culture of Strongyloides and serological methods like ELISA. However demonstration of antibodies by ELISA does not distinguish between acute and chronic infections. Endoscopy directed biopsy and radiological findings also help in the diagnosis of Strongyloidiasis [3].
The associated mortality rate in Strongyloidiasis is more than 50% in post-transplant patients [10], and as high as 87% in hyperinfection [3]. Personal hygienic measures like proper hand hygiene, prevention of contact with infected soil, proper disposal of sewage and health education will help to prevent infection with Strongyloides [3]. Immunocompromised patients should have knowledge about the disease, its mode of spread, symptoms and its prevention. Health education can be given to them with the help of a care manager who assists the patient individually, helping him to adopt behaviors and lifestyles suitable to his health condition, and encouraging greater self-sufficiency in the monitoring of the parameters through the knowledge of the disease which would help to prevent the disease and its associated morbidity and mortality in these patients [11].
Conclusion
Strongyloidiasis should be considered as a differential diagnosis in immunocompromised patients with gastrointestinal and respiratory symptoms and early diagnosis would prevent the mortality associated with hyperinfection or disseminated strongyloidiasis. However, our patient was treated successfully for Strongyloides.
Acknowledgement
We thank our technicians Mr. Rajesh Naidu and Mr. Bharat Bhushan for their technical support.
Conflicting Interest
None
6921
References
- Siddiqui AA, Berk SL (2001) Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040-1047.
- Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 17: 208-217.
- Vadlamudi RS, Chi DS, Krishnaswamy G (2006) Intestinal strongyloidiasis and hyperinfection syndrome. ClinMol Allergy 4: 8.
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, et al. (2013) Strongyloides stercoralis: Global Distribution and Risk Factors. PLoSNegl Trop Dis 7: e2288.
- Manocha H, Dua S, Chander Y, Tailang M (2014) Cryptosporidiosis, whether it is more prevalent in Southern India. Trop Parasitol 4: 125-127.
- Nagamani K,Rajkumari A; Gyaneshwari (2001) Cryptosporidiosis in a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol 19: 215-216.
- Kalawat U, Abhijit C, Jaya P(2013) Strongyloidesstercoralis infections in patients attending a tertiary care Hospital: Series of seven cases. IJSR 2: 362-363.
- Carvalho EM, Da Fonseca Porto A (2004) Epidemiological and clinical interaction between HTLV-1 and Strongyloidesstercoralis. Parasite Immunol 26: 487-497.
- Naidu P, Yanow SK, Kowalewska-Grochowska KT1 (2013) Eosinophilia: A poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol 24: 93-96.
- Montes M,Sawhney C, Barros N (2010) Strongyloides stercoralis: there but not seen. CurrOpin Infect Dis 23: 500-504.
- Cecere A, Scicchitano P, Zito A, Sassara M, Bux F et al. (2014) Role of Care Manager in Chronic Cardiovascular Diseases Ann Gerontol Geriatric Res 1: 1005.






