Adryan Perez1, Lawrence Guan2, Kyle Sutherland2 and Chuanhai Cao1,2*
1Department of Pharmaceutical Science, College of Pharmacy, University of South Florida, Tampa, FL 33612, USA
2USF-Health Byrd Alzheimer’s Institute University of South Florida, Tampa, FL 33613, USA
Corresponding Author:
Chuanhai Cao
Department of Pharmaceutical Science, College of Pharmacy
University of South Florida, Tampa, FL 33612, USA
Tel: 8133960742
E-mail: ccao@health.usf.edu
Received date: December 18, 2015 Accepted date: January 12, 2016 Published date: January 19, 2016
Citation: Perez A, Guan L, Sutherland K, et al. Immune System and Parkinson’s Disease. Arch Med. 2016, 8:2. doi: doi number
Keywords
Parkinson’s disease, Immune therapy, Immunomodulation, Alpha synuclein, Immunotherapy, Immune modulation
Introduction
Parkinson’s disease (PD) was established as a medical condition many years after James Parkinson noted a resemblance in cases amongst his older patients in his 1817 publication “An Essay on the Shaking Palsy” [1]. It is characterized as a progressive neurodegenerative movement disorder that results from the death of dopaminergic neurons in the substantia nigra [2]. Presently, there is no known cure for PD and drugs used to treat it are focused on suppressing symptoms instead of preventing the onset or the progression of the disease. The most common drugs used to treat PD are dopamine or dopamine derivatives such as L-DOPA, which have been found to cause levodopainduced dyskinesias (LIDs) following chronic treatment [3]. Many other treatments have been developed and applied in the clinical setting including natural products, vaccines, antibody treatments, receptor agonists, and physical treatments but have so far been unsuccessful in preventing or slowing the disease.
Modern medicine is extending the average lifespan of the population making the prevalence of PD increasingly impactful on society. Today PD affects about one million patients in the United States and more than seven million worldwide with a mean age of diagnoses occurring in the seventh decade of life [4]. PD pathology is currently classified as either idiopathic or hereditary, however only 5-10% of PD cases are inherited and attributed to a specific gene mutation [5]. The higher prevalence of PD found in the older population compared to the younger population lends support to the hypothesis that its pathogenesis is correlated with immunosenescence and an aging body.
Patients suffering from PD may begin to display symptoms many years before the disease is clinically diagnosed. Currently there is no pinpoint diagnostic test for PD, therefore diagnoses relies on the clinical detection of two of the four cardinal signs of PD which include resting tremor, bradykinesia, rigidity, and postural imbalance [6]. In addition to the physical symptoms, PD patients commonly display non-motor symptoms such as depression, lack of motivation, passivity, and dementia [7]. After PD is diagnosed, patients live an average of 15 years before death occurs [8]. The prodromal phase associated with PD can last many years and can be attributed to the slow rate of decline of the immune system with age [9]. Accurate prognosis of PD relies on the discovery of a biomarker present in the immune system attributed to the disease that can be modified to prevent disease pathophysiology.
This review article will explore changes in the immune system of PD patients that lead to an imbalance between the innate and adaptive immune systems in order to hopefully uncover the etiology and pathophysiology of the disease.
The Pathological Markers of PD
The clinical detection of PD in patients lies along a broad spectrum starting from normal to severely impaired motor and non-motor functions. Unfortunately, the presence of motor skill impairments may not be used as appropriate biomarkers due to the significant amount of neurodegeneration already present at this stage in disease pathophysiology [10]. The discovery of an early biomarker will allow patients to receive treatment at the inception of disease progression consequently minimizing dopaminergic neuronal loss and increase the success of therapeutic efforts. The presentday absence of an early defining stage in PD development creates urgency for the identification of specific biomarkers that can be used in studies focused on preventing the development of PD.
One promising method for the detection of a useful biomarker is research on the exposome. The exposome consists of all human environmental exposures from conception onwards [11], and is a biological record of the body’s interaction with disease and environmental exposures. Through the completion of the exposome, it will be possible to determine environmental causes that lead to the degradation and deregulation of the immune system occurring with age leading to the discovery of useful biomarkers to identify the PD in its premature stages of progression. Though at this time research is limited in this area, and it will take further studies to fully utilize the exposome with regards to the prognosis of PD.
Currently, the most commonly used pathological marker for PD is the aggregation and misfolding of the endogenous protein α-synuclein, which is efficiently metabolized by the body at the monomeric level and when there is an appropriate balance between innate and adaptive immunity. After the immune system ages and is no longer able to properly metabolize α-synuclein, the protein becomes main component of the toxic aggregates termed Lewy bodies (LBs). These toxic aggregates pose a problem to the declining immune system. As the aggregates continue to grow due to their prion-like nature, they are marked as foreign by the innate immune system leading to an influx of phagocytic cells to the area which are unsuccessful in engulfing and destroying the large oligomers at this stage in disease advancement. The continued inability to metabolize the large toxic aggregates causes a chronic inflammatory process that eventually leads to neuronal death. After cell death occurs, the apoptotic factors released contribute to the further recruitment of inflammatory elements. Thus, the increase of inflammatory factors and the progression of this immune cascade in PD patients can potentially be used as early biomarkers for disease detection and immune modulation.
One of the hallmarks of PD is the death of dopaminergic neurons (DA). DA neurons have recently been demonstrated to have characteristics of traditional antigen presenting cells (APCs), such as dendritic cells. DA neurons can process foreign ovalbumin (OVA), a 385 amino acid protein that is found in egg whites. This sequence is cleaved to SIINFEKL peptide in vitro and presented by traditional APC, and by major histocompatibility complex class-I (MHC-I). This ability demonstrates the DA’s ability to process foreign protein and display derived peptides on the cell surface [12]. Neuronal MHC-I can subsequently trigger immune response and T-cell-mediated cytotoxic attack and degradation.
Red blood cells (RBCs) contain 99% of the α-synuclein present in the blood [13]. In a person with a healthy immune system, once red blood cells reach the end of their lifespan, they lyse and the α-synuclein is released into the blood stream where it becomes ably metabolized. The red blood cell life cycle occurs continuously throughout life and once α-synuclein becomes released into a weak and unbalanced immune system the protein is not sufficiently metabolized, leading to a continual increase in the amount of α-synuclein present in the blood [14,15].
As shown in Figure 1, the accumulation of α-synuclein monomers in the blood increases the chances of the protein interacting to form a higher order molecule. The free α-synuclein monomers circulating in the blood that are not metabolized remain undetected by the immune system. Once these monomers bind to other monomers, forming a stable dimer or oligomer of higher order structure, they are recognized as foreign by the immune system and illicit an inflammatory immune response. These interactions between α-synuclein monomeric units lead to the formation of various conformers that have been observed in the brain [16]. Thus, early detection of high concentrations of α-synuclein in the peripheral blood may serve as a potential biomarker of PD before the protein invades and affects the central nervous system.
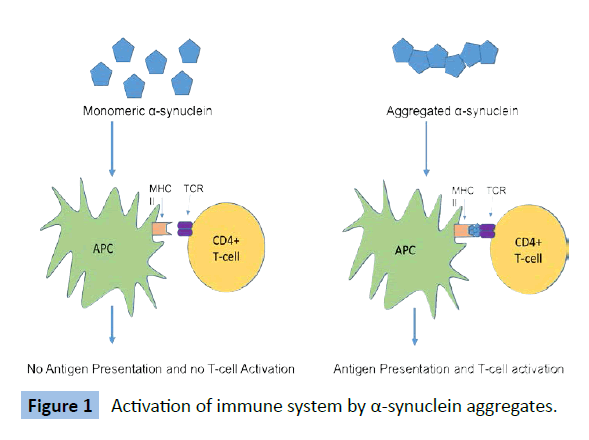
Figure 1: Activation of immune system by a-synuclein aggregates.
As the inflammatory state of the PD brain persists, the bloodbrain- barrier becomes increasingly permeable, allowing the entry of T-cells. The α-synuclein causes induction of microglial major histocompatibility complex class II (MHCII) and subsequent antigen presentation [17]. We hypothesize that this progressive sensitization of T-cells through the course of disease will gradually increase rate of degeneration of dopaminergic neurons that exhibit MHCI presentation of α-synuclein derived peptide.
The Immune System in PD
The immune system is categorized into innate and adaptive immunity. Innate immunity is present at birth and is a nonspecific response to defend the body from infection [18]. Unlike the innate immune system, the adaptive immune system has the ability to mount a specific immune response against foreign antigens. Although both the innate and adaptive immune systems can be affected by aging, the biggest toll is placed upon the adaptive immune system [19].
The declining of the immune system is considered a normal part of the aging process. Immunosenescence is clearly demonstrated through the involution of the thymus, which plays a major role in the development of T cells in the adaptive immune system and the increased presence of diseases amongst the elderly population [20]. Due to a weakened immune system, the elderly exhibit immunological tolerance through a decreased response to vaccination which leads to the need for vaccines of higher doses in order to elicit an appropriate response from the adaptive immune system [21]. These changes indicate the immune system in older individuals loses its strength through the inability to maintain the critical balance present at younger ages increasing susceptibility to diseases.
A key feature of a healthy immune system is the appropriate recognition between “self” and “non-self” when mounting immune responses. PD patients have demonstrated an increase in heterogeneity of immune responses leading to inappropriate autoimmune reactions towards endocrine insulin and astrocytical S100B [22]. These immune reactions may represent the neurodegenerative processes leading to disease pathology occurring in the brain. The inappropriate responses can also potentially occur against other endogenous proteins related to the disease such as alpha synuclein, leading to a myriad of other immune system alterations.
Changes in the innate immune system
A key change found in the aging innate immune system is a decrease in the amount of dendritic cells [23,24]. Dendritic cells (DC) are involved in capturing and processing foreign antigens, expressing lymphocyte co-stimulatory molecules, and secreting cytokines to elicit an immune response from the adaptive immune system [25]. Studies have found aged DCs to exhibit altered functions such as the impaired ability to induce T cell proliferation [26] and to increase levels of proinflammatory cytokines [27]. The changes made in dendritic cells may clarify the role played by the innate immune system to the overall development of immunosenescence and provide insight on possible immune modulating treatments [23].
Microglia are resident macrophages present in the CNS that serve as a neuroprotective medium through the defense against infectious diseases, inflammation, trauma, ischemia, brain tumors, and neurodegeneration [28]. Once presented with an antigen, microglia become activated quickly to eliminate the threat. The activation of microglia takes place before any other tissue damage or cell death takes place so it is used as a measure of ongoing neuronal injury [29]. In PD, an increased amount of activated microglia in the brain is associated with the active disease state and is believed to actually cause an effect leading to further neurodegeneration by inducing cell death in dopaminergic neurons [30]. Once microglia become activated by a stimulus such as α-synuclein, they may remain activated and respond atypically to subsequent stimuli thereby enhancing inflammation-induced oxidative stress [31].
A key functional component of dendritic cells and microglia are the toll-like receptors (TLRs) of the innate immune system present on the cell surface. Toll-like receptors function to recognize constituents of invading pathogens, which induces the production of proinflammatory cytokines and activates a response from the antigen-specific adaptive immune system [32]. The activation of the adaptive immune system via TLRs may contribute to the disparity present between the innate and active immune system present in PD patients leading to further disease progression. The role played by TLRs in the inflammatory cascade makes them a potential target as a viable biomarker for the development of future PD treatments.
Changes in the adaptive immune system
Consequential changes occur in the adaptive immune system with age that inhibit the body’s ability to maintain the critical balance necessary to maintain a disease-free environment. A notable change occurs to hematopoietic stem cells (HSCs) which function to give rise to all cells present in the blood. Previous research has shown that the phenotypic and functional properties of HSCs change during ontogeny [33]. Due to the defects accumulated by HSCs with age, their population declines because of their reduction in regeneration ability. The modifications to HSCs leads to the subsequent decrease in production of naïve T cells and naive B cells in the bone marrow and thymus contributes to the disruption of balance between the innate and adaptive immune system responsible for keeping the body disease free at younger ages [34]. Due to the decreased population of naïve cells in the older population, the ability of the immune system to recognize new antigens subsequently diminishes [35].
The purpose of immunomodulation in treating PD and other diseases caused by the immune system is to bring the immune system back to its balanced state present in earlier stages of life. By identifying changes occurring in the immune system of PD patients, it would be possible to prevent the disease or stop its progression at an earlier stage. Targeting these changes within the immune system, such as those presented in Table 1, may help to slow the neurotoxic environment that is created by over activation and inflammation of the immune system. Parkinson’s disease is an age related disorder, and it is possible that a cure may likely be found in a young and healthy immune system.
| |
Role in Immune System |
Role in PD |
| TNFα. |
Cell signaling protein involved in triggering apoptotic cell death and inflammation |
Present in elevated levels in PD patients[36,37]; mediates cell death in a responsive population of DA neurons by activating brain microglia[38,39]; stimulates production of nitrogen oxide lead. |
| IFNγ |
Involved in both innate and adaptive immune system; activates macrophages and causes expression of Class II Major histocompatibility molecules (MHC) |
Present in elevated levels in PD patients[40]; involved in death of DA neurons by up regulating microglial activity[40,41]. |
| IL-1β |
Pro-inflammatory cytokine; involved in cell proliferation, differentiation, and apoptosis |
Present in elevated levels in PD patients[37]; sustained pro-inflammatory levels has toxic effects in the substantianigra[42] |
| TGF-β1 |
Anti-inflammatory cytokine |
Conjunction with IL-6 generates high amounts of Th17 causing additional inflammation[43] |
| IL-6 |
Pro-inflammatory cytokine; involved in acute phase response |
Increased levels correlated with severe depression, fatigue and cognitive difficulties in PD patients[44]; acceleration of muscle catabolism/poor physical performance[45] |
| IL-10 |
Anti-inflammatory cytokine; Up regulation of humoral response, Down regulation of cellular response |
Aids in protecting against LPS-induced loss of dopaminergic neurons[46]; decreased levels found in aged brain[47] |
| IL-4 |
Stimulate differentiation of naïve helper T cells to Th2 cells; Stimulate active B cells |
Increased concentrations in PD patients[48] |
| Dendritic Cells (DCs) |
Antigen-presenting cells; link between innate and adaptive immune systems |
DC levels decrease as PD symptoms become more severe[49]; Vaccination may be an effective treatment against a-synuclein[50] |
Table 1: Summary of potential immune targets in PD.
Conclusion
The correlation between an aging immune system and Parkinson’s disease must not be ignored. As patients continue to age, the immune system gradually undergoes changes that increase the risk of developing PD, and everyone is susceptible as 90-95% of the cases are sporadic. By continuously studying the evolving immune system, we come closer to being able to intervene and restore it to its balanced form present at younger ages. It is crucial to identify and adjust the disparity between the innate and adaptive immune system in order to prevent disease onset and progression. Aiding in this goal would be the discovery of an early biomarker present in the primitive stages of disease development. Also critical in future research is the further role characterization and elucidation of underlying mechanisms of the many immune cells related to disease pathogenesis. The identification of an earlier marker and more in depth information about the contributing immune cells would allow for the combinatorial use of drugs used presently along with methods of immunomodulation to return the immune system to its healthy and balanced state.
8460
References
- Shulman JM, De Jager PL, Feany MB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 6: 193-222.
- Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci 28: 57-87.
- Niccolini F, Rocchi L, Politis M (2015) Molecular imaging of levodopa-induced dyskinesias. Cell Mol Life Sci 72: 2107-2117.
- Yao SC, Hart AD, TerzellaMJ (2013) An evidence-based osteopathic approach to Parkinson disease. Osteopathic Family Physician 5: 96-10.
- Samii A, Nutt JG, Ransom BR (2004) Parkinson's disease. The Lancet 363: 1783-1793.
- de Lau LM, Breteler MM (2006) Epidemiology of Parkinson's disease. Lancet Neurol 5: 525-535.
- Fahn S (2003)Description of Parkinson's Disease as a Clinical Syndrome. Annals of the New York Academy of Sciences 99: 1-14.
- Lees AJ, Hardy J, Revesz T (2009) Parkinson's disease. Lancet 373: 2055-2066.
- Hawkes CH (2008) The prodromal phase of sporadic Parkinson's disease: does it exist and if so how long is it? MovDisord 23: 1799-1807.
- Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, et al. (2013) Biomarkers in Parkinson's disease (recent update). NeurochemInt 63: 201-229.
- Vrijheid M (2014) Theexposome: a new paradigm to study the impact of environment on health. Thorax 69: 876-878.
- Cebrian C (2014) MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nature communications 5: 3633-3633.
- Barbour R, Kling K, Anderson JP, Banducci K, Cole T, et al. (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5: 55-59.
- Lee PH, Lee G, Park HJ, Bang OY, Joo IS, et al. (2006) The plasma alpha-synuclein levels in patients with Parkinson's disease and multiple system atrophy. J Neural Transm (Vienna) 113: 1435-1439.
- El-Agnaf OM, Salem SA, PaleologouKE, Curran MD, Gibson MJ, et al. (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 20: 419-425.
- Gould N, Mor DE, Lightfoot R, Malkus K, Giasson B, et al. (2014) Evidence of native α-synuclein conformers in the human brain. J BiolChem 289: 7929-7934.
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, et al. (2013) MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33: 9592-9600.
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197-216.
- Castelo-Branco C, Soveral I (2014) The immune system and aging: a review. GynecolEndocrinol 30: 16-22.
- Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, et al. (2009) Thymic involution and immune reconstitution. Trends Immunol 30: 366-373.
- McElhaney JE, Dutz JP (2008) Better influenza vaccines for older people: what will it take? J Infect Dis 198: 632-634.
- Wilhelm KR, Yanamandra K, Gruden MA, Zamotin V, Malisauskas M, et al. (2007) Immune reactivity towards insulin, its amyloid and protein S100B in blood sera of Parkinson's disease patients. Eur J Neurol 14: 327-334.
- Della Bella S (2007) Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clinical Immunology 122: 220-228.
- Shodell M, Siegal FP (2002) Circulating, Interferon-Producing Plasmacytoid Dendritic Cells Decline During Human Ageing. Scandinavian Journal of Immunology 56: 518-552.
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245-252.
- Gupta S (2014) Role of dendritic cells in innate and adaptive immune response in human aging. ExpGerontol 54: 47-52.
- Panda A (2010) Age-Associated Decrease in TLR Function in Primary Human Dendritic Cells Predicts Influenza Vaccine Response. The Journal of Immunology 184: 2518-2527.
- KreutzbergGW (1996) Microglia: a sensor for pathological events in the CNS. Trends in Neurosciences 19: 312-318.
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, et al. (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis 21: 404-412.
- Shrestha R, ShakyaShrestha S, MillingtonaO2, Brewer J3, BushellT2 (2014) Immune responses in neurodegenerative diseases. Kathmandu Univ Med J (KUMJ) 12: 67-76.
- Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M (2013) Neuroimmunological Processes in Parkinson's Disease and their Relation to a-Synuclein: Microglia as the Referee between Neuronal Processes and Peripheral Immunity. ASNNeuro.
- ReuvenEM, Fink A, ShaiY2 (2014) Regulation of innate immune responses by transmembrane interactions: lessons from the TLR family. BiochimBiophysActa 1838: 1586-1593.
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, et al. (2005) Cell intrinsic alterations underlie hematopoietic stem cell aging. ProcNatlAcadSci U S A 102: 9194-9199.
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K (2013) Causes, consequences, and reversal of immune system aging. J Clin Invest 123: 958-965.
- GoronzyJJ, Weyand CM (2005) T cell development and receptor diversity during aging. Current Opinion in Immunology 17: 468-475.
- Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Advances in Research on Neurodegeneration.
- Reale M (2009) Peripheral cytokines profile in Parkinson’s disease. Brain, Behavior and Immunity 23: 55-63.
- McGuire SO (2001) Tumor Necrosis Factor a Is Toxic to Embryonic Mesencephalic Dopamine Neurons. Experimental Neurology 169: 219-230.
- Qin L, Wu X, Block ML, Liu Y, Breese GR, et al. (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453-462.
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, et al. (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27: 3328-3337.
- Delgado M (2003) Inhibition of Interferon (IFN) ?-induced Jak-STATActivation in Microglia by Vasoactive Intestinal Peptide: INHIBITORY EFFECT ON CD40, IFN-INDUCED PROTEIN-10, AND INDUCIBLE NITRIC-OXIDE SYNTHASE EXPRESSION. Journal of Biological Chemistry 278: 27620-27629.
- Leal MC, CasabonaJC, Puntel M, PitossiFJ (2013) Interleukin-1 and tumor necrosis factor: reliable targets for protective therapies in Parkinson's Disease? Front Cell Neurosci 7: 53.
- Korn T, Bettelli E, Oukka M, KuchrooVK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27: 485-517.
- Lindqvist D (2013) Cerebrospinal fluid inflammatory markers in Parkinson’s disease – Associations with depression, fatigue, and cognitive impairment. Brain, Behavior and Immunity 33: 183-189.
- Scalzo P, Kümmer A, Cardoso F, Teixeira AL (2010) Serum levels of interleukin-6 are elevated in patients with Parkinson's disease and correlate with physical performance. NeurosciLett 468: 56-58.
- Arimoto T, Choi DY, Lu X, Liu M, Nguyen XV, et al. (2007) Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantianigra. Neurobiol Aging 28: 894-906.
- Ye SM, Johnson RW (2001) An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 9: 183-192.
- Brodacki B (2008) Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFa and INF? concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neuroscience Letters 44: 158-162.
- Ciaramella A, Salani F, Bizzoni F, Pontieri FE, Stefani A, et al. (2013) Blood dendritic cell frequency declines in idiopathic Parkinson's disease and is associated with motor symptom severity. PLoS One 8: e65352.
- UgenKE (2015) Evaluation of an alpha synuclein sensitized dendritic cell based vaccine in a transgenic mouse model of Parkinson's disease. Human Vaccines &Immunotherapeutics.







