Alsharydah AM1, Alanazi AH2, Alsuhaibani SS1, Alsayyah AA3 and Al-Jehani HM1,2*
Department of Radiology and interventional radiology, King Fahad Hospital of the University, University of Dammam, Saudi Arabia
Neurosurgery Department, King Fahad Hospital of the University, University of Dammam, Saudi Arabia
Department of Pathology, King Fahad Hospital of the University, University of Dammam, Saudi Arabiay
- *Corresponding Author:
- Hosam Al-Jehani
Department of Radiology and interventional radiology, King Fahad Hospital of the University, University of Dammam, Saudi Arabia
E-mail: hjehani@uod.edu.sa
Tel: +9668966743
Fax: +9668966743
Received date: December 03, 2015 Accepted date: December 22, 2015 Published date: December 28, 2015
Introduction
Recently, quality control of medical care is becoming increasingly more important. In hospitals, departments are required to meet certain quality standards stated by hospital administration, and internal audit is a valuable instrument that can be used to obtain and evaluate treatment outcome data. Even publication is expected to get increasingly controlled by standardized guidelines. This rigor serve the surgical specialties in different ways, as it gives an indication of the level of care provided and specific information about operational risks encountered. It also allows for the scientific analysis of factors that might be linked to a better practice and to improve patient care, and allow comparing those results with other benchmarks pertaining to surgeons and institutions [1].
Pediatric brain tumor (PBT) is the second most common form of childhood cancer, and account for approximately 20% of childhood cancer diagnoses [2]. Because of the availability of aggressive combination therapies, including surgical resection, chemotherapy, radiotherapy, and peripheral blood stem cell transplantation, overall improvement in outcome has been observed over the last decade [3-5].
Comprehensive approach to these patients is being implemented more frequently. For example, although the initial imaging of any solitary pediatric CNS tumor, might not affect the initial strategy of surgical intervention (gross total vs. subtotal resection) histopathological diagnosis and follow up imaging in instrumental in guiding the whole post-operative care, as well as the prognosis [6,7].
Lately, several advances and improvements in the histopathological diagnostics and differentiation of central nervous system (CNS) tumor subtypes allowed prognostication that was not previously available. To ensure a uniform and standardized approach, CNS tumors have been re-classified according to the internationally accepted World Health Organization (WHO) classification of brain tumors [8]. Therefore, clinical protocols combine imaging of PBT and histopathology, and discuss the ramifications of such combination on outcome of treatment of CNS tumors among the pediatric population.
The primary aim of this study is to perform an internal quality assessment of pediatric brain tumor surgery done by the neurosurgery department in the University of Dammam at the Kingdom of Saudi Arabia. Secondly, this study aims to contribute to the accumulating data concerning outcome in pediatric neurosurgery, in order to establish institutional practice benchmarks.
Methods
The study was conducted at King Fahad Hospital of the University (KFHU) a tertiary care university teaching hospital with 600 beds in the Eastern Province of Saudi Arabia. After obtaining our local institutional research approval, we conducted a retrospective cohort study of pediatric patients with the diagnosis of PBT in the last 10 years, from December 2005 till February 2015. During this period, there was one dedicated pediatric neurosurgeon in our hospital, no pediatric oncologist nor a neuro-oncologist, and no dedicated diagnostic neuroradiologist. All patients were younger than 16 years at the time of the first surgery. Data collected from chart review, collecting data that include demographics, initial lab investigations, disease comorbidities, management plan, and outcome. In addition, revising all radiological images, radiological reports, pathological specimens and pathological reports. We excluded all DNR (do not resuscitate) patients and, patients diagnosed with pseudo-tumors (Cysts). Data was entered and analyzed using SPSS program version 22. All results are displayed as mean and SD. Categorical variables were compared with the 2 × 2 or Fisher’s Exact Test. Quantitative variables were compared using the Kruskall-Wallis nonparametric test. The results were supposed to be statistically significant for p-values <0.05.
Results
A total of 62 charts were reviewed. After exclusions, 45 patients were included in the final analysis.
The mean age of patients is 7 years (SD ± 4. 5). 62% were males and 38% were females. 76% were Saudi in nationality. The family history of PBT was not documented in 55% of charts.
Review of diagnostic imaging revealed that 45% of patients were operated on the basis of CT alone. Cranial MRI was obtained the in the remaining 55%. Of those, Diffusion-Weighted imaging (DWI) was obtained in only 20% of patients. Spinal MRI was done in scarcely whether preoperatively (8.4%), and postoperatively (3.3%) respectively.
Intraoperative frozen section histopathological examination was obtained in 18% of cases. Permanent histopathological examinations revealed that most of PBT is primary glial in origin in 78%. Of those the majority (59%) were low-grade lesions, 26% of those were astrocytomas and 8.7% were ependymomas. Glioblastoma represented 4.3% of our cohort. The remainder were primitive tumors including PNET, Medulloblastoma, Pinealblastoma and hemangioblastoma (Table. 1). Only 47% of tumors were subjected to further genetic analysis. There was rare dissemination to the spinal cord (2%). Similarly, CSF cytological analysis was conducted in 2% of patients.
| Tumor type |
Total (%) |
| GBM |
2 (4.3%) |
| Astrocytoma |
12 (26.6%) |
| Ependymoma |
4 (8.7%) |
| Germinoma |
3 (6.5%) |
| Hemangioblastoma |
1 (2.2%) |
| Craniopharyngioma |
5 (11.1%) |
| PNET/Medulloblastoma |
5 (11.1%) |
| Pinealblastoma |
1 (2.2%) |
| Oligodendroglioma |
2 (4.3%) |
| Details not available |
10 (22.2%) |
| Total |
45(100%) |
Table 1: Distribution for each type of PBT.
In total, surgical resection was the primary step in management in all patients. 78% of patients suffered no morbidity and good long-term outcome and with a 10-year-progression survival rate of 64%. Overall survival data is lacking since 80% of patient have no long-term data on record (Figure. 1). No intraoperative mortality was encountered. However post-surgical mortality during the hospital stay was 4.4%. The Mean length of hospital stay was 22 days, with most patients receiving adjuvant therapy in other institutions.
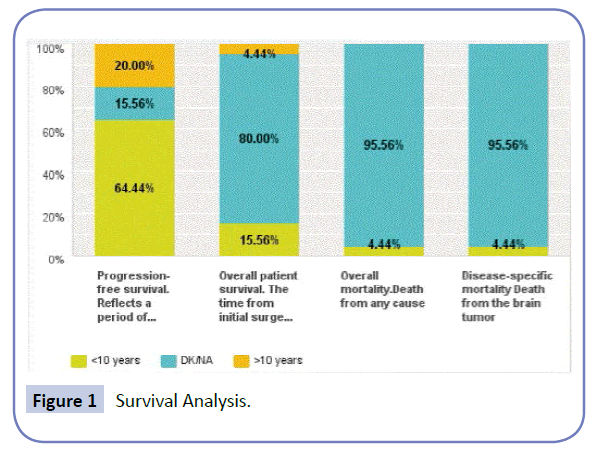
Figure 1 Survival Analysis.
Discussion
There is significant improvement in the outcome of treatment of pediatric CNS tumors, owing to the improvement in the inter-disciplinary care provided for these patients. This included improvement in clinical diagnosis, imaging, operative techniques and adjuvant therapies provided.
Quality-of-care metrics and indicators of patient care in pediatric Neuro-oncology surgery are hardly available, and a review of the literature shows noticeable differences in the reported findings owing to the heterogeneity of criteria experienced when selecting the type of surgery for each patient, when evaluating the extent of resection, or when defining surgical complications and their severity [9].
In this study, we evaluated the practice in our center when approaching patients with the diagnosis of PBT. Following our model of care, the neurosurgery department was the most responsible for caring for the patients, coordinating all aspects of care. With this, there is extensive collaboration with a multidisciplinary team including, pediatrics, radiology and, histopathology departments as well as the oncology department of other institutions for adjuvant therapy. There is a constant concern of missing a step of this process and owing to the variability of practice backgrounds in our hospital, the chances of this are relatively high given that a standardized protocol or a flow chart for care does not exist.
Nonetheless, 78% of patients had their treatment without neurological morbidity after first surgery. This is generally in line with the reported morbidity of 33% and 54% neurological complications in recent studies [10,11]. Overall mortality in our patients with PBT undergoing surgery was found to be 4.4% which is comparable to the reported rates ranging from 0% to 20% in other studies [12,13].
It is well known that high-volume hospitals and high-volume surgeons lead to lesser morbidity and mortality [14]. That is why we always raise a concern given the low volume status in our hospital. But the attempts to establish benchmarks for our practice are vital to maintaining safety of the treatment we provide to PBT patients, e.g., mandating detailed documentation of factors known to affect patients with PBT and their families. To this effect we have to move to more regional complimentary network of hospitals that serve PBT to provide primary and adjuvant therapies in a streamlined fashion. We emphasize the value of the human factor too, with dedicated pediatric neurosurgeons, neuro-oncologist, and neuro-radiologists creating a specialized multidisciplinary team acquainted with the updates rapidly occurring in the field. This will improve the care that the patients should receive in our center and help maintain this benefit for the future and most importantly establish metrics for us to continue to improve our care and compare our performance to that of the rest of the practice institutions.
Conclusion
Despite the lack of a sub-specialized multidisciplinary team caring for patients with PBT, the overall surgical mortality rate in KFUH was (4.4%) with a 22% morbidity, which is keeping with those of high-volume neurological centers. Nonetheless, logistical improvements and investing in the specialties involved in caring for patients with PBT will allow maintaining this outcome and create quality metrics that can be used in the future to gauge our progress.
Acknowledgement
The authors wish to thank Dr. Faisal Al-Ousi and Dr. Mohammed AlAftan for their assistance in data collection.
IRB approval of this study (IRB-2014-01-357).
7825
References
- Albright AL, Pollack IF, Adelson PD, Solot JJ (1999) Outcome data and analysis in pediatric neurosurgery. Neurosurgery 45:101-106.
- Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, et al. (2004) Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet 364: 2097-105.
- Duffner PK (2010) Risk factors for cognitive decline in children treated for brain tumors. Eur J PaediatrNeurol 14:106-115.
- Cohen K, Broniscer A, Glod J (2001) Pediatric glial tumors. Curr Treat Options in Oncol 2: 529-536.
- Kalifa C, Grill J (2005) The therapy of infantile malignant brain tumors: current status? J Neurooncol 75:279-285.
- Rosenzweig I, Bodi I, Selway RP, Crook WS, Moriarty J, et al. (2010) Paroxysmal ictal phonemes in a patient with angiocentricglioma. J Neuropsychiatry ClinNeurosci22:123 E18-20.
- Ma X, Ge J, Wang L, Xia C, Liu H, et al. (2010) A 25-year-old woman with a mass in the hippocampus. Brain Pathol 20:503-506.
- Louis DN, Ohgaki H, Wiestler OD,Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. ActaNeuropathol114:97-109.
- Brell M, Ibánez J, Caral L, Ferrer E (2000) Factors influencing surgical complications of intra-axial brain tumors. ActaNeurochir (Wien) 142: 739-750.
- Sandri A, Sardi N, Genitori L, Giordano F, Peretta P, et al. (2006) Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome. Childs NervSyst 22: 1127-1135.
- Young HK, Johnston H (2003) Intracranial tumors in infants. J Child Neurol 19:424-430.
- Sinson G, Sutton L, Yachnis A, Duhaime A, Schut L (1994) Subependymal giant cell astrocytomas in children. PediatrNeurosurg 20:233-239.
- Cowan JA, Dimick JB, Leveque JC, Thompson BG, Upchurch GR, et al. (2003) The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery 52: 48-54.
- Smith E, Butler W, Barker F (2004) Craniotomy for resection of pediatric brain tumors in the United States, 1988 to 2000: effects of provider caseloads and progressive centralization and specialization of care. Neurosurgery 54: 553-565.