Geetha R.V1* Anitha Roy2
1Assistant professor, Department of Microbiology, Saveetha Dental College, Velappanchavady, Chennai-77
2Assistant professor, Department of Pharmacology, Saveetha Dental College, Velappanchavady, Chennai.-77
- Corresponding Author:
- Mrs. Geetha R.V
Assistant professor, Department of Microbiology
Saveetha Dental College, Velappanchavady, Chennai-77
E-mail: rgeetha2010@yahoo.in
Received Date: 01-08-2012; Accepted Date: 21-08-2012
Citation: Geetha R.V* Anitha Roy “In Vitro Evaluation of Anti bacterial Activity of Ethanolic root extract of Glycyrrhiza glabra on Oral microbes” Int. J. Drug Dev. & Res., October-December 2012, 4(4): 161-165.
Copyright: © This is an open access paper distributed under the copyright agreement with Serials Publication, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords
Glycyrrhiza glabra, Anti bacterial, Disc diffusion, MIC,MBC.
Introduction
Herbal medicines have been used for many years. There are innumerable types of indigenous plants that have been used by people for centuries in the treatment of many ailments. The history of such usage is long and well documented. [1] Researchers found that people in different parts of the world tended to use the same or similar plants for the same purposes. The development of drug resistance in human pathogens against commonly used antibiotics has necessitated a search for new antimicrobial substance from other sources including plants. [2] Herbs with medicinal properties are useful and effective source of treatment for various diseases. Currently many studies are being conducted to know these herbs in depth. Screenings of medicinal plants for antimicrobial activities are important for finding potential new compounds for therapeutic use. There have been numerous reports of the use of traditional plants and natural products for the treatment of oral diseases. [3] The present study was to evaluate the antibacterial activity of ethanolic root extract of Glycyrrhiza glabra on selected oral microorganisms.
Glycyrrhiza glabra, also known as liquorice and sweet wood, is native to the Mediterranean and certain areas of Asia. It is a perennial herb which possesses sweet taste. The main taproot, which is harvested for medicinal use, is soft, fibrous, and has a bright yellow interior. [4] It was one of the most widely known medicines in ancient history, and records of its use include Assyrian tablets of around 2000 BC and cortisone has been found useful for arthritis and allergies. In addition licorice has been used for mild Addison’s disease and other adrenal insufficiencies, such as hypoglycemia. [5] Licorice also acts like the hormone, ACTH, causing sodium retention, potassium depletion, and water retention. The herb contains glycyrrhizin, glycyrrhetinic acid, flavonoids, asparagine, iso-flavonoids, and chalcones. The glycoside, glycyrrhizin has a similar structure and activity as the adrenal steroids. [6,7] It also possess good anti bacterial, [8 anti fungal, [9] anti oxidant, [10] antitussive, [11] hepatoprotective [12] and anti inflammatory activity. [13] Historically, the dried rhizome and root of this plant were employed medicinally as an expectorant and carminative. It is used for treating upper respiratory ailments including coughs, hoarseness, sore throat and bronchitis.
Materials and Methods
Plant material
The ethanolic root extract of Glycyrrhiza glabra was obtained from Green Chem Herbal Extract & Formulations. Bangalore.
Test microorganisms
Bacterial strains used were Streptococcus mutans, Streptococcus sanguis, Streptoccoccus mitis Streptococcus salivarius, Lactobacillus acidophilus. The organisms were isolated using selective media Mutans -Sanguis agar [ Hi media M977], Mitis- Salivarius Agar [Hi media M257] and Lactobacillus MRS agar [Hi media M641] and maintained in nutrient agar slope at 4°C in department of Microbiology, Saveetha Dental College.
Methodology
The extracts were prepared in the following concentrations in sterile water. 2.5mg/ml, 5mg/ml and 10mg/ml. 50μl of extract of different concentrations were loaded on sterile filter paper discs measuring 6mm in diameter, so that the concentration of the extract on each disc was 125μg, 250μg and 500 μg respectively. The discs were dried and kept aseptically
Screening of antibacterial activity [Disc diffusion technique]
Broth culture of the bacterial strains compared to Mac Farland’s standard [14,15] 0.5 were prepared. Lawn culture of the test organisms were made on the Muller Hinton agar [MHA-Hi media M1084] plates using sterile cotton swab and the plates were dried for 15 minutes. Filter paper discs loaded with different concentrations of the extract were placed on the respective plates. The plates were incubated at 37°C overnight and the zone of inhibition of growth was measured in millimeters. Standard antibiotic discs of amoxicillin (30mcg/disc) and PenicillinG (30mcg/disc) were used as positive control. All the tests were done in triplicate to minimize the test error.
Determination of minimum inhibitory concentration
Macro broth dilution or tube dilution method was done to determine the Minimum inhibitory concentration (MIC) of the extracts [16,17]. A series of two fold dilution of each extract ranging from 8mg/ml to 0.125mg/ml was made in Muller Hinton broth as specified by National Committee for Clinical Laboratory Standards (NCCLS, 1998). 100μl of standard inoculum of the bacterial strains matched to 0.5 Mc Farland’s standard were seeded into each dilution. Two control tubes were maintained for each test batch. These included antibiotic control (tube containing extract and growth media without inoculum) and organism control (tube containing the growth medium and the inoculum) .The tubes were incubated at 37°C for 24 hours and checked for turbidity. MIC was determined as the highest dilution (that is, lowest concentration) of the extract that showed no visible growth.
Minimum Bactericidal Concentration (MBC)
The MBCs were determined by selecting tubes that showed no growth during MIC determination; a loop full from each tube was sub cultured onto Muller Hinton agar plates and incubated for further 24 hours at 37°C. The least concentration, at which no growth was observed, was noted as the MBC
Result and Discussion
The antibacterial activity of the extracts at different concentrations was screened by disc diffusion technique and the zone of inhibition was measured in mm diameter. The results are given in the table 1. The minimum inhibitory concentration [MIC] and minimum bactericidal concentration [MBC] were also determined for the extracts and the results are given in table 2.
| Extract |
Conc [µg] |
Zone of inhibition [in mm diameter] |
| |
|
B1 |
B2 |
B3 |
B4 |
B5 |
| |
125 |
15 |
12 |
10 |
14 |
13 |
| |
250 |
19 |
15 |
13 |
17 |
16 |
| |
500 |
22 |
19 |
16 |
20 |
20 |
| Penicillin G |
30mcg/disc |
24 |
21 |
22 |
26 |
21 |
| Amoxycillin |
30mcg/disc |
25 |
23 |
20 |
24 |
22 |
B1- Streptococcus mutans, B2- Streptococcus sanguis, B3- Streptococcus salivarius, B4- Streptococcus mitis, B5- Lactobacilus acidophilus
Table 1: Anti bacterial activity of ethanolic root extract of Glycyrrhiza glabra
| Extract |
|
MIC/MBC values [mg/ml] |
| |
|
B1 |
B2 |
B3 |
B4 |
B5 |
| Ethanolic |
MIC |
2 |
2 |
4 |
4 |
4 |
| MBC |
2 |
2 |
8 |
4 |
4 |
B1- Streptococcus mutans, B2- Streptococcus sanguis, B3- Streptococcus salivarius, B4- Streptococcus mitis, B5- Lactobacilus acidophilus
Table 2: MIC and MBC of ethanolic root extract of Glycyrrhiza glabra
The ethanolic extract was more effective against Streptococcus mutans with a zone of inhibition of 22mm diameter (at conc500 μg.) and was less effective against Streptococcus salivarius with zone of inhibition of 16 mm (at conc.500 μg.) and among the other bacterial species studied Streptococcus mitis and Lactobacillus acidophilus showed a zone of inhibition of 20mm diameter (at conc. 500 μg.) both and Streptococcus sanguis showed inhibition zone of 19 mm diameter (at conc.500 μg.).
The MIC and MBC values of ethanolic extract was found to be low compared to aqueous extract. The ethanolic extract was found to have Low MIC and MBC values of 2mg/ml & 2mg/ml for both Streptococcus mutans and Streptococcus sanguis. With Streptococcus salivarius, ethanolic extract showed a higher MIC and MBC value of 4mg/ml & 8mg/ml and for Streptococcus mitis and Lactobacillus acidophilus it was 4mg/ml and4mg/ml. The lower MIC and MBC value is an indication of high effectiveness of the extract whereas higher MIC and MBC indicates the less effectiveness of the extract.
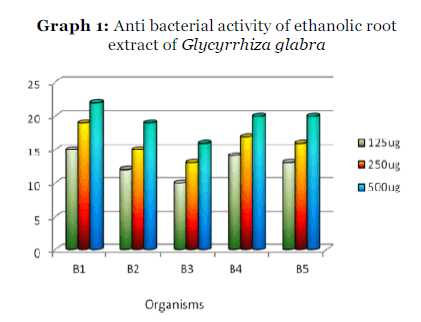
Graph 1: Anti bacterial activity of ethanolic root extract of Glycyrrhiza glabra
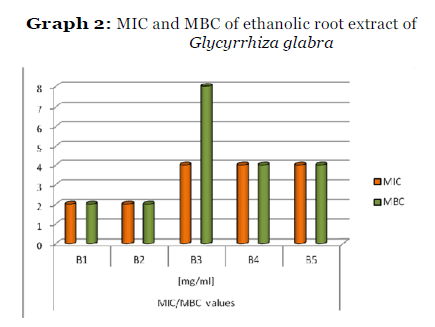
Graph 2: MIC and MBC of ethanolic root extract of Glycyrrhiza glabra
Oral diseases are major health problems with dental caries and periodontal diseases among the most important preventable global infectious diseases. Oral health influences the general quality of life and poor oral health is linked to chronic conditions and systemic diseases. The association between oral diseases and the oral microbiota is well established. Dental caries is a microbial disease that result in the destruction of mineralized tissue of the teeth. Streptococcus mutans is the potent initiator and leading cause of dental caries world wide .18 It is considered to be the most cariogenic of all of the oral Streptococci. Another organism that is important in the development of caries is Lactobacillus acidophilus. This bacteria is not important in the initiation of caries but in the continuation. The present study was to evaluate the antibacterial activity of ethanolic root extract of Glycyrrhiza glabra against caries causing organisms. The results obtained from our study shows that ethnolic extract has got a very good antibacterial activity against the selected oral pathogens.
Conclusion
The present results therefore offer a scientific basis for traditional use of root extract of Glycyrrhiza glabra on oral pathogens. The anti-bacterial activities could be enhanced if active components are purified and adequate dosage determined for proper administration. The use of herbs in dentistry should be based on evidence of effectiveness and safety. Although there may be benefits to using herbal medicines in the practice of dentistry, we really do not know much about them. With additional research, there definitely will be a niche for herbal treatments in dentistry
Conflict of Interest
NIL
Source of Support
NONE
5128
References
- Sheetal verma and S.P.Singh., Current and future status of herbal medicines. Veterinary world, Vol.1(11): 347 – 350.
- Si-Yuan Pan, Si-Bao Chen, Hong-Guang Dong, Zhi- Ling Yu, Ji-Cui Dong, Zhi-Xian Long,Wang-Fun Fong, Yi-Fan Han, and Kam-Ming Ko.,New perspectives on Chinese herbal medicine research & development. Evidence Based Complementary and alternative medicine volume 2011.,11 pages.
- Enzo A. Palombo., Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Review article. Evidence – Based complementary and Alternative Medicine Vol 2011,Article ID680354,15 pages
- Olukoga A, Donaldson D. Historical perspectives on health. The history of liquorice: the plant, its extract, cultivation, and commercialisation and etymology. J R Soc Health 1998; 118:300-304
- A.AAl-Qarawi, H.A.Abdel Rahman, B.H.Ali, Liquorice (Glycyrrhiza glabra and the adrenal kidney -pituitary axis in rats. Food and chemical toxicology, vol 40: issue 10 pages 1525-1527
- Fan Y. G.; Shi Z. Q.; He B. L.; Extraction separation and application for glycyrrhizic and glycyrrhetic acid. Natural product Research and Development. 1996, 8, 93‐99
- Yang L.; Liu Y. L.; Lin S. Q.; The determination of flavonoid in 6 kinds of licorice root by HPLC.Acta Pharmaceutics Sinica. 1990,25,840-848
- Manoj M. Nitalikar, Kailas C. Munde, Balaji V. Dhore, Sajid N. Shikalgar Antibacterial Activities of Glycyrrhiza glabra Root Extract ,International Journal of PharmTech Research Vol.2, No.1, pp 899‐901
- Tharkar P.R.,Tatiya A.U., Shinde P.R ., Surana S.J., Patil U.K .,Antifungal Activity of Glycyrrhiza glabra Linn. And Emblica Officinalis Gaertn. by Direct Bioautography Method International Journal of PharmTech Research .,Vol.2, No.2, pp 1547-1549, April-June 2010
- Morteza-Semnani K, Saeedi M, Shahnavaz B,Comparison of antioxidant activity of extract from roots of licorice (Glycyrrhiza glabra L.) to commercial antioxidants in 2% hydroquinone cream. J Cosmet Sci. 2003 Nov-Dec;54(6):551-8.
- Anderson DM, Smith WG. The antitussive activity of glycyrrhetinic acid and its derivatives. J.Pharm.Pharmacol. Jul 1961; 13:396‐404.
- Kang HE, Lee MG, Hwang SJ, Kim SC, Lee CH, et al. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase‐II enzymes. Am J Physiol Gastrointest Liver Physiol. Feb 2009;296(2):372‐81
- Okimasu E, Moromizato Y, Watanabe S, et al. Inhibition of phospholipase A2 and platelet aggregation by glycyrrhizin, an anti inflammation drug. Acta Med Okayama 1983;37:385‐391.
- Collins, CH and Lyne, P.M 1976.Microbiological methods, London, Butterworths and co.288p
- Betty A.Forbes., Daniel F.Sahm., Alice S.Weissfeld. Bailey & Scott’s Diagnostic Microbiology 11th edition Mosby page 229 – 257
- Connie R.Mahon., George Manuselis., Saunder’s Diagnostic Microbiology 2nd Edition.
- J. G. Colle., A. G. Faster., B. P. Marmion. A. Simmons. Practical Microbiology [Mackie & Mc Cartney] 14th edition page 851 – 852
- L. P. Samaranayake.,Brain M.Jones., Essential Microbiology for Dentistry. Second edition page 217 – 223.








