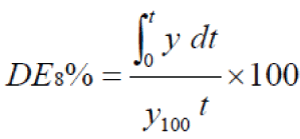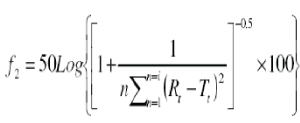Key words
|
| |
| Diltiazem HCl, Matrix and layered matrix tablets, Controlled released |
| |
Introduction
|
| |
| Oral ingestion has long been the most convenient and commonly employed route of drug delivery due to its ease of administration and flexibility in the design of the dosage form. There are many ways to design modified release dosage forms for oral administration and one of them is multilayered matrix tablet(1). One to three multi layered matrix tablet is a drug delivery device, which comprises a matrix core containing the active solute and one, or more barriers (modulating layers) incorporated during tabletting process (2). The modulating layers delay the interaction of active solute with dissolution medium, by limiting the surface available for the solute release and at the same time controlling solvent penetration rate (3). In the device, the coat layers prevent the water penetration through the protected core for some duration. After this phase during the subsequent dissolution process, the swollen barriers erode and surface available for drug release slowly increases. In this way the decrease of delivery rate due to increase in diffusion path length (saturation effect) is counter balanced by the simultaneous increase of the area available for drug release (4, 5). Thus by combining a time-dependent control of the hydration rate of the device with the reduction of tablet surface exposed to the dissolution medium, it is feasible to achieve a linear release profile. |
| |
| Diltiazem HCl is a calcium channel blocker used for the treatment of chronic stable angina pectoris and for angina pectoris caused by a coronary arterial spasm and systemic hypertension. It is an acidic salt of basic drug having a pKa value 7.7 and the molecule is freely soluble in water. Although 90% of an orally administered dose of diltiazem HCl is absorbed, only 40% of the oral dose reaches systemic circulation in an unchanged form. The mean absolute bioavailability of diltiazem HCl in normal subjects ranged from 33 to 44% and uniformly absorbed throughout the gastro intestinal tract and posses short halflife 3- 4.5hrs, which dictates dosing three times a day.(6,7) Hence it is appropriate to formulate in a controlled release, once a day dosage form. This study is to investigate the usefulness of layered matrix tablets in providing oral controlled drug delivery of highly water soluble diltiazem HCl. |
| |
MATERIALS AND METHODS
|
| |
| Diltiazem HCl (Divis Laboratories Hyderabad, India), Guar Gum (Medium Viscosity grade) (Natural gum form H.B.Gum, Kalol, India), Cellulose derivatives, Sodium Carboxy Methyl Cellulose (SCMC) (high viscosity grade) (Reliance Cellulose Product, Hyderabad, India), and Di calcium phosphate was procured from (Loba Chime Pvt. Ltd. Mumbai, India). All other materials were of analytical grade. |
| |
|
Preparation of diltiazem HCl matrix coregranules and barrier layer granules
|
| |
| The drug and polymers for the matrix tablets and layered matrix tablets were passed through sieve number18, before their use in the formulation. The matrix formulations were prepared with 30%, 40% and 50% guar gum in the matrix core and coded as D1, D2 and D3 respectively. Matrix core granules were prepared by wet granulation procedure using non-ionic guar gum, di-calcium phosphate as diluent, starch paste 10% as binding agent. The cohesive mass obtained was passed through sieve number 18, dried at 50°C for 1hr in a tray dryer. The granules were lubricated with a mixture of talc and magnesium stearate. |
| |
| The barrier layer containing anionic SCMC, were prepared by wet granulation technique. The polymer SCMC and 10% starch paste were mixed well and the resulting wet mass was passed through sieve no 18 and dried at 30°C for an hour. To increase the flow property of the SCMC granules and to prevent its adhesion to die and punches the granules were lubricated with talc and magnesium stearate. |
| |
|
Compression of layered matrix tablets
|
| |
| Matrix tablets were coded as D1, D2 and D3. The composition of formulation used in the study containing 90mg of diltiazem HCl. The layered matrix tablets were prepared by using different combinations of drug loaded matrix core granules and barrier layer granules. Initially the volume of die cavity was adjusted equivalent to total weight of layered matrix tablets 350mg, 400mg and 450mg respectively. Then pre weighed amount of anionic SCMC granules equivalent to bottom layer 50mg, 75mg, and 100mg were taken and coded as L1, L2 and L3 correspondingly, placed in the die cavity and uniformly spreaded. The upper punch was lifted up and 250mg of matrix core granules were placed over the bottom layer of SCMC granules in the die cavity and slightly compressed. The remaining volume of die cavity was filled with pre weighed amount of SCMC granules equivalent to top layer 50mg, 75mg and 100mg respectively. Finally compressed on a rotary compression machine (Riddhi, Ahmedabad, India). The hardness of tablets was adjusted to 5- 6kg/cm2. |
| |
|
in vitro Drug Release Study
|
| |
| Drug release was studied using a dissolution apparatus type 2 (Lab India, DISSO 2000, Mumbai, India) with a shaft at a speed of 50 rpm. To study the effect of dissolution medium, drug release was studied in 900-mL HCl of pH 1.2 for 2 hours and then the pH of medium was replaced with pH 7.4 at 37±1°C for 12h. Samples were collected at specific time intervals and assayed by a UV spectrophotometer (Elico, Model SL-150, Mumbai, India.) at a wavelength of 237 nm. During the drug release studies, the tablets were observed for physical integrity. The experiments were repeated three times and results were taken as average of three test readings with standard deviations. The accuracy and precision of the standard curve was sufficiently accurate, with a validated linearity for determination of drug in dissolution media. |
| |
|
Analysis of release data
|
| |
| The description of dissolution profiles has been attempted using different release models. The data were evaluated according to the following equations. |
| |
| Zero order: Mt = Mo+ Kot |
| |
| First order: ln Mt = ln Mo+ K1t |
| |
| Higuchi model: Mt = KH √t |
| |
| Korsmeyer –Peppas model: Mt/Mo = Kktn |
| |
| Where Mt is the amount of drug dissolved at time t, Mo the initial amount of drug, K1is the first order release constant, Ko the zero order release constant, KH the Higuchi rate constant, Kk the release constant and n is the diffusional release exponent indicative of the operating release mechanism. The correlation coefficient (r2) was used as an indicator of the best fitting, for each of the models considered. |
| |
| The following equation was used to calculate the other dissolution parameters D.E8% and MDT mean dissolution data (8) |
| |
 |
| |
| Mean dissolution time (MDT) is a measure of the rate of the dissolution process calculated from the amount of drug released to the total cumulative drug. To compare the results of dissolution tests of different formulations: Dissolution efficiency (D.E)(9)after 8hr of release test was used. |
| |
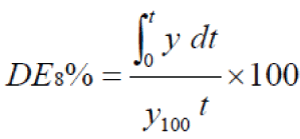 |
| |
| The similarities between two dissolution profiles were assessed by a pair wise model independent procedure similarity factor (f2). |
| |
|
Similarity factor
|
| |
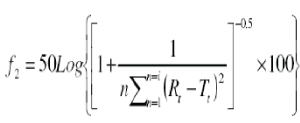 |
| |
| Where n is the sampling number, Rt and Tt are the percent dissolved of the reference and test products at each time point t. Where Wt is the optional weight factor (normally taken as 1). f2 values should be close to 100. In general f2 values higher than 50 (50-100), show the similarity of dissolution profiles (8). |
| |
|
in vivo Bio availability Study
|
| |
| Eight male volunteers, aged between 23 and 25 years and weighing between 62 and 68kg participated. Studies were conducted with permission from the institutional ethical committee. The protocol of study of was approved by the Human Ethical Committee, University College of Pharmaceutical Sciences, Kakatiya University, India, with reference number UCPSc/BA/2009-06. which compiled ethical to the Helsinki Declaration. The purpose of the study was fully explained to the volunteers and an informed written consent was obtained from each volunteer. This study was performed in a two-way crossover design with a washout period of one week between two phases. No other drugs were taken during the study period. The subjects were randomly divided into two groups. A light breakfast was served to all volunteers on the study day after overnight fasting, followed by administration of D3L3 to one group and Dilzem SR tablet to another group with a glass of water after half an hour of breakfast. No food was allowed until 4 hrs after dosing. Approximately 5 ml of blood samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 and 24hrs. Samples were collected by antecubital vein via a hypodermic syringe rinsed with dilute heparin. The samples were centrifuged for 10mins and plasma was stored at -20°C until analyzed by the modified method of Al-Saidan et al . (10) |
| |
|
High-Performance Liquid Chromatography Determination of Diltiazem HCl in Human Plasma
|
| |
| Each of plasma (0.5mL) accurately measured into a 10-mL glass tube and added 100µl of verapamil HCl as an internal standard, (0.5 µg) along with 0.2ml of water, 0.25ml of 1.0 M dipotassium hydrogen phosphate, and 3mL of cyclohexane: diethyl ether in 2:1 ratio was added and shaken for 20 minutes. The organic phase was separated and transferred to another test tube containing 0.5mL of 0.01M HCl. These tubes were shaken to extract the drug and internal standard (verapamil HCl) into the aqueous phase. 20µl of supernant was directly injected into the HPLC column. Peak area and peak height were computed. The retention time of diltiazem HCl and Verapamil HCl were 3.0 min and 3.41min respectively. |
| |
|
Pharmacokinetics data analysis
|
| |
| The data obtained from Diltiazem HCl in Plasma concentration time profile, were analyzed for each subjects using non compartmental method. The Pharmacokinetics parameters were estimated by using KINETICATM Soft ware (INNA Phase Corp., 2000) |
| |
|
Statistical Analysis
|
| |
| The data obtained was statistically analyzed using a using Graph pad prism version 4. (Graph pad prism Software, Inc). Paired t-test was used for comparison of all the Pharmacokinetic parameters. A value of P<0.05 was considered to be significant and results were expressed as mean± SD. |
| |
Results and Discussion
|
| |
|
Physico chemical characterization of matrix and layered matrix tablets
|
| |
| The physical parameters such as hardness was ranked from 5.92±0.10 to 6.10±0.02 kg/cm2, thickness ranked from 3.02 ±0.01mm to 6.02±0.02mm, friability ranked from 0.251±0.02% to 0.868±0.01%, mass of the tablets ranged from 250.01±0.01 to 451.0 ±1.68 (mg) and drug content in the range of 97.3± 2.01% to 102.2± 2.15% of the formulations. The hardness and thickness of the tablets were increased as the amount of anionic SCMC layers was increased. The hardness of layered matrix tablets tended to increase and the friability decreased. |
| |
|
in vitro drug release and kinetic characteristics
|
| |
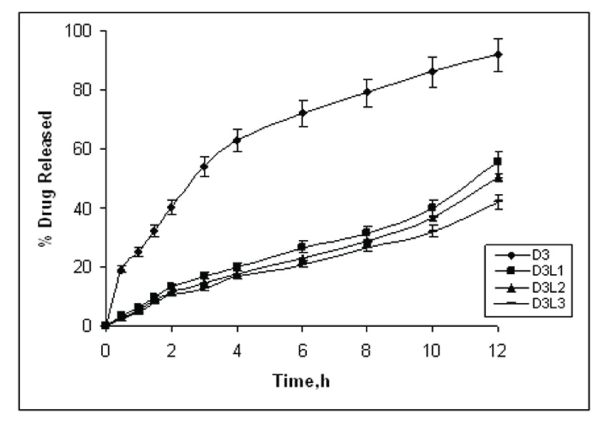
| Apparent drug release prolongation could be attained for the layered matrix tablets. The percentage drug release from formulations D3, D3L1 and D3L3 ranged from 98.76±1.08 %, 55.6±1.87% and 42.06±1.98% Thus on the basis of drug release data, it is evident that as thickness of the polymer layer increased the rate of drug release was found to be decreased. 100 mg of SCMC layered tablets (D3L3) provided the desired release rate compared to 50mg and 75mg layered matrix tablets (D3L1 and D3L2), hence linearization has been achieved. The higher corelation coefficient (r2) indicated a superiority of the dissolution profile to the mathematical equations. The matrix tablets (D3), shows higher (r2) values for the first order kinetics (r2 =0.996) Table 2, indicated that diltiazem HCl released from matrix tablets followed first order kinetics. It was observed that the edges of layers were rounded off due to slight erosion of swollen barrier layers of SCMC. The results indicated that anionic and pH dependent polymer SCMC does not fully hydrate when placed in 0.1 N HCl, but when the dissolution media was replaced with pH 7.4 it showed rapid hydration and forms a viscous gel layer that forming a loose porous on the surface of the tablet facilitating the matrix core seeping of diltiazem HCl from the surfaces of the matrix core resulting in constant delivery of the drug [10]. For studying the mechanism of drug release from the tablets, the dissolution data was fit into korsmeyer’s and peppas equation. The diffusional exponent values (n) for the matrix tablets shows less than 0.5 indicated that it follows Fickian diffusion, as shown in Table 1. And for layered matrix tablets were greater than 0.5 and less than 1 indicated non- Fickian diffusion, where as in case of D3L2 and D3L3 (n >1) follows super case type II. The values of the kinetics constant (k) were in accordance with the values of n, the diffusional exponent; with k having lower values when the transport mechanism was case II and higher values for matrix tablets that released the drug by Fickian diffusion. Thus diltiazem HCl released from the middle guar gum matrix core could be modified by the delayed diffusion from the barriers applied on both surfaces of the matrix core. The diltiazem HCl released from layered matrix tablets tended to prolong the release with the amount of barrier layers applied. Hence the release was modulated by applying barrier layers. |
| |
| Mathematical analysis of kinetic data obtained revealed that drug release from matrix tablets was mainly attributed to Fickian diffusion. While SCMC layered matrix tablets shows either non- Fickian diffusion or super case type-II mechanism as shown in Table 2. The higher (r2) values; signifies that the developed layered matrix tablets show zero order or case II release. The independent statistical evaluation, i.e. Dissolution efficiency (DE8%), mean dissolution time (MDT) and paired tests is similarity factor (f2). The data is shown in Table 2. MDT and DE8% values of D3 and D3L3 formulations were found to be 4.17h, 16.45h and 86.02%, 63.55% respectively. MDT is increased, while DE8% decreased, indicating that the release of diltiazem HCl is slower, which is attributed to increased in the thickness of barrier layers (SCMC) on the matrix core. |
| |
| The results of f2 values showed similar dissolution profiles for plain matrix tablets Where as anionic SCMC layered matrix tablets showed dissimilar dissolution profiles data shown in Table 1, with that of commercial diltiazem HCl (Dilzem SR®) sustained release tablets. The layered matrix tablets (D3L3) was selected as a suitable formulation for the bioavailability study in human volunteer, since the results of in-vitro release of D3L3 formulation showed slower drug release in both pH medias (pH 1.2 and pH 7.4) and is shown in figure 1. |
| |
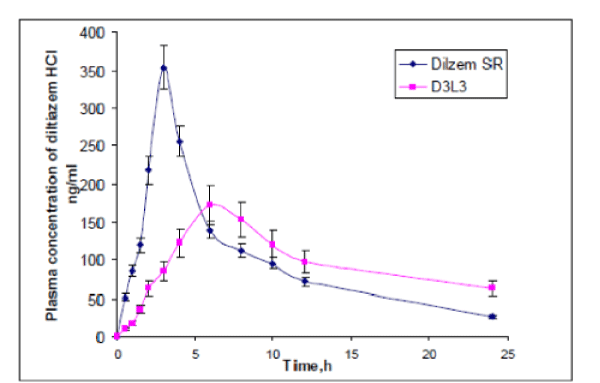
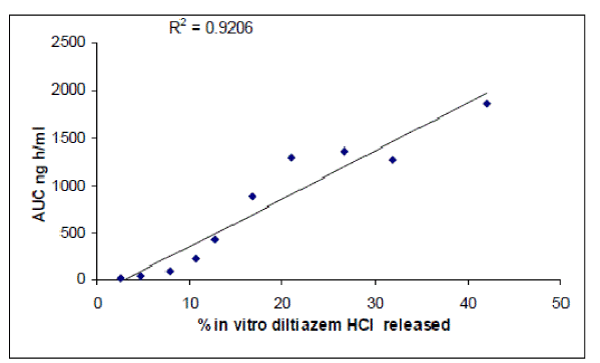
| The plasma concentrations of diltiazem HCl at different time intervals following oral administration of both the formulations (Dilzem SR and D3L3) are shown in figure 2. The mean pharmacokinetics parameter of diltiazem HCl is shown in Table 2. The peak serum concentration (Cmax) and time to reach peak concentration (Tmax) of Dilzem SR and D3L3 were found to be 360.82± 5.65, 172.41±5.11ng/ml and 3.125±0.12hrs and 6.0hrs of diltiazem HCl respectively. The lower Cmax of formulation D3L3, prolonged the Tmax. The Mean residence times (MRT) of Dilzem SR and D3L3 formulation were 11.08±0.23hrs and 22.33±1.92hrs. A significant difference was observed for the time to reach peak concentration Tmax and Mean residence time (MRT) between these two formulations. Thus the lower Cmax of MLT -06, prolonged the Tmax, an increase in AUC0- shows unaltered bioavailability. It indicated that the release of diltiazem HCl from D3L3 formulation is slow and it may be attributed due to the longer residence time in the GIT, thereby providing a controlled and effective absorption of diltiazem HCl. in vitro-in vivo correlation was done by plotting percentage drug absorbed versus in vitro drug release. The plot was linear for the layered matrix tablet D3L3 with a correlation coefficient of 0.920, indicating high correlation is shown in figure 3. This in vitro-in vivo correlation is of great use in developing of controlled release tablets (11). diltiazem HCl quickly with in 3.125±0.12hrs, resulting in faster absorption. There by it produces high peak concentration Cmax with earlier Tmax, when compared to layered matrix D3L3. Guar Gum dispersed matrix core layered with 100mg of SCMC on both the surfaces as barrier layers were found to be useful to achieve controlled release of diltiazem HCl throughout the gastrointestinal tract. Results show that guar gum as matrix core and cellulose derivative (SCMC) as layers is suitable and promising for production of controlled release layered matrix tablets. These dosage forms can be developed on large scale using layered tablet press. |
| |
Acknowledgement
|
| |
| The authors are thankful to Divis laboratories Hyderabad, India, H.B Gums, Kalol India and Reliance cellulose products, Hyderabad, India for generous donating the gift samples of diltiazem hydrochloride, guar gum and Sodium Carboxy Methyl Cellulose respectively. And greatful to kakatiya universitywarangal. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |