Keywords
Turbinaria conoides; Ethyl acetate fraction; 4-NQO; Antigenotoxicity; Anti-inflammatory
Introduction
Human populations are under threat due to the exposure of various endogenous and environmental agents like pesticides, metals, polycyclic aromatic hydrocarbons (PAHs), solvents, and alkylating agents along with therapeutic compounds including antitumor and antibiotics [1]. As a result, the cellular macromolecules get damaged including DNA and leads to genetic alteration, endothelial dysfunction, gastrointestinal dysfunction, tissue injury and inflammation. Inflammation is a main precursor for the following diseases such as rheumatoid arthritis, atherosclerosis, inflammatory bowel diseases, pleuritis and nephritis. There are conventional drugs used to ameliorate those diseases. However, it is too expensive, offer only temporary relief and often elicits undesirable side effects. Therefore, inhibition of genotoxicity and their subsequent inflammatory events might be remedy for treating those diseases through marine resources [2]. Many drugs from marine source have potential to eradicate the hazardous substance induced oxidative stress by scavenging the free radicals which are known to possess antioxidant, antigenotoxic, antiinflammatory and antinociceptive properties [3].
Turbinaria conoides (J. Agardh) Kuetz. brown algae coming under the order of Fucales and belongs to the family Sargassaceae. Traditionally, it is being used as a remedy for the children’s fever, as a fertilizer, insect repellent, pesticide and bacterialcidal [4]. T. conoides possessed important phytochemicals such as fucosterol, sulfated polysaccharides fucoidan, neutral glucan, guluronic and alginic acid [5]. Apart, it contained digestible proteins along with mineral salts (K, Ca, and Fe) and polyunsaturated fatty acids with wealthy source of dietary fiber. Rich source of iodine shows play an immense role in enhancing the food quality and biochemical homeostasis [6,7]. T. conoides and T. ornata collected from Gulf of Mannar of Southeastern coast of India were also reported to have the antioxidant activity due to the presence of total phenolic content in ethyl acetate fraction [8]. Sodium alginate was one of the bioactive compounds present in brown seaweeds which exhibit various biological effects like removal of heavy metal, antitumour and anti-inflammatory property [9]. Most marine plants having biologically active constituents are resided mainly in the polar fraction. Moreover, brown alga being used as a traditional medicine which is essentially required a scientific validation through experimentally and clinically to find out their efficacy. Thus, the present study was undertaken to determine the antigenotoxic and antiinflammatory activities of ethyl acetate fraction of ethanol extract of T. conoides in animal model.
Materials and Methods
Chemicals
Indomethacin, Giemsa stain, May-Grunwald stain, Aspirin and 4-nitroquinoline-1-oxide (4-NQO) were obtained from Sigma-Aldrich, USA. Phosphate buffered saline, acetic acid and DMSO were obtained from Hi-media, Mumbai, India. All solvents used in this study were of analytical grade.
Seaweed collection and preparation of solvent fractions
The brown seaweed of T. conoides was collected from intertidal zone of Mandapam, Gulf of Mannar, Souch-east coast of India. The algae samples (1 kg) were washed with fresh tap water and followed by distilled water to remove salt and other debris along with necrotic parts. Samples were made into small pieces, shade dried and then powdered using a mixer grinder. The powdered sample was stored in a polyethylene bag at room temperature. The shade dried powder 250 g was immersed in a 2 L of two conical flasks with each 1.25 L of 95% ethanol and left for 24 h under constant stirring. After the 24 h, transfer from conical flask to Soxhlet apparatus and extracts was collected in bottom flask at 40°C. This was repeated twice with 2.5 L of 95% ethanol. Final volume of 4.5 ml ethanol extract was brought out into 25 g (10%) with rotary evaporator [10]. The ethanol extract was transfer into a separating funnel and partitioned between distilled water and hexane with 1:6 ratio. This mixture was thoroughly mixed for 15 minutes and the hexane fraction (HF) was collected 1.4 L after 1 h incubation. Similarly, aqueous layer 250 ml was further fractionated with dichloromethane (DMF) and ethyl acetate (EAF). All the fractions were concentrated in rotary evaporator. The yield of the fractions was 20%, 16%, and 52% respectively. The aqueous fraction (AF) was lyophilized and obtained 12% of yield. Among fraction of T. conoides, EAF of was used in-vivo experiment based on their In-vitro antioxidant potential.
Experimental animals
Both sexes of Swiss albino mice (20-25 g) and Wistar albino rat (120-180 g) were obtained from King Institute, Chennai, India. The animals were housed in standard environmental conditions (12 h light/12 h dark; 22 ± 2°C) for one week prior to the experiments to acclimatize to the laboratory conditions. They were allowed free access to tap water and pelleted rodent diet. The animal care and experimental protocols were accordance with the Guidelines of the Committee for Control and Supervision of Experiments on Animals (CPCSEA), India.
Experimental design for antigenotoxic potential of T. conoides
Either sex of Swiss albino mice was divided into six groups with five mice each. The concentrated ethyl acetate fraction (EAF) was dissolved in 15% DMSO and administered by oral gavage to the mice for 5 consecutive days. Control group 1 fed with 15% DMSO by orally. Group 2 received 450 mg/kg bw of EAF. Group 3 received with 7.5 mg/kg bw of 4-NQO by intraperitoneally. Groups 4, 5 and 6 received respective test doses 75, 150, and 450 mg/kg bw of EAF for five consecutive days plus 4-NQO (7.5 mg/kg bw; i.p.) on sixth day. After 24 hours of 4-NQO treatment, all the mice were sacrificed by cervical dislocation. The bone marrow cells were flushed out into phosphate buffered saline (PBS) and prepared for the micronucleus assay based on the protocol reported in our previous publication [11]. For the micronucleus assay, each animal (experimental/control), 2,500 polychromatic erythrocytes (with or without micronuclei) and a corresponding number of normal chromatic erythrocytes (NCEs) were scored under a light microscope.
Carrageenan induced paw edema in rats
Wistar albino rats were divided into five groups with each 5 rats. Control (15% DMSO), three test doses of EAF (75, 150, and 450 mg/kg) and indomethacin as a positive control (10 mg/kg in distilled water) were administrated orally. After 1 hour, 0.1 mL of 1% solution of freshly prepared carrageenan in normal saline was injected in the sub plantar surface of the right hind paw of the rats. The paw volume was measured (as cm) before and after injection of carrageenan at the time periods of 1, 2, 4 and 8 hours by mercury displacement Plethysmograph. The degree of acute inflammation was expressed as centimeter with % inhibition of paw volume by standard and EAF of T. conoides over the control value [12].
Acetic acid induced writhing and tail immersion method in mice
The writhing and tail immersion method was carried in mice per the method of Ferreira et al. [13], Kumar and Shankar [14] respectively. Swiss albino mice were divided into five groups with each five mice. Control group (15% DMSO), three test doses (75, 150, and 450 mg/ kg of EAF) and Aspirin (100 mg/kg) as a positive control were administrated orally. All the test doses and standard drug were fed one hour before of chemical stimulus. The writhes were induced by i.p. injection of 1% acetic acid (v/v, 10 mL/kg) in all the groups except control. After ten minutes, the number of writhe was recorded over a period of ten minutes. A writhe is indicated by abdominal constriction and full extension of hind limb. The tail flick response was measured after administration of test and standard drugs at the period of 60, 120 and 180 minutes. Tail flick latency difference or mean increase in latency after drug administration was used to indicate the analgesia produced by test and standard drug.
Statistical analysis
Results were presented as mean ± standard deviation for five animals of each group. Statistical analyses were performed by one-way ANOVA using SPSS Software Version 16.0. The Student-Neuman- Keuls (SNK) test was applied to assess for differences among the groups. Values of P ≤ 0.05 were considered to be significant.
Results
The antigenotoxic potential of ethyl acetate fraction of T. conoides against 4-NQO induced micronucleated polychromatic erythrocytes (MnPCEs) in mice bone marrow cells were evaluated and presented in Figure 1. The well-known genotoxin, 4-NQO enhanced the frequency of MnPCEs was about 4.1-fold over the control value, 16.0 ± 0.71 MnPCEs/2500 PCEs. Ethyl acetate fraction alone treated group showed no changes in the frequency of MnPCEs (17.0 ± 0.10, P>0.05). However, pretreatment of ethyl acetate fraction of T. conoides along with 4-NQO was observed to be significantly reduced the frequency of MnPCEs ranging from about 52 to 18%. The reduction was greatest at 450 mg/kg bw (about 72%) and lowest at 75 mg/kg bw (about 20%). Hence, dose 150 mg/kg bw was also effectively reduced the frequency of the MnPCEs (about 57%) induced by 4-NQO.
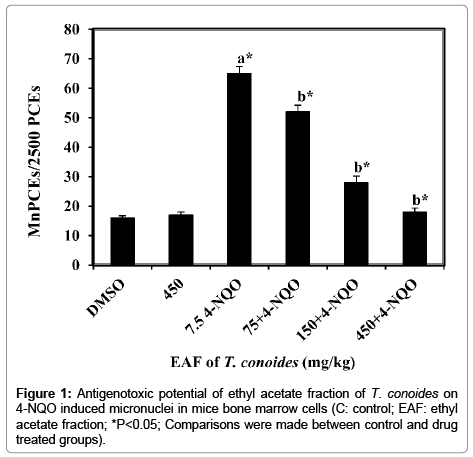
Figure 1: Antigenotoxic potential of ethyl acetate fraction of T. conoides on 4-NQO induced micronuclei in mice bone marrow cells (C: control; EAF: ethyl acetate fraction; *P<0.05; Comparisons were made between control and drug treated groups).
Anti-inflammatory potential of ethyl acetate fraction of T. conoides was evaluated against carrageenan induced paw edema in Wistar albino rats. The increased paw edema in rat was measured at time interval of 1, 2, 4 and 8 hours (Table 1). Paw edema in the control group was observed to be enhanced about 0.74 ± 0.10 to 0.44 ± 0.05 at time interval of 1-8 hrs. In both indomethacin and ethyl acetate fraction of T. conoides were observed to be decrease the paw edema significantly based on the dose along with time interval. The reduction rate of paw edema by indomethacin was about 49-64% with respect to the control group. However, ethyl acetate fraction of T. conoides was also effectively reducing the carrageenan induced paw edema. At the dose of 150 mg/kg bw, the reduction of the paw edema was observed to be 32-36% at the time intervals of 1-8 hrs. The dose 75 mg/kg bw treated group showed the least reduction of paw edema about 15%. The significant reduction of paw edema by ethyl acetate fraction of T. conoides was found to be as equal as standard anti-inflammatory drug, indomethacin.
| Treatment groups |
Oral dose (mg/kgbw) |
Paw edema at different time intervals (h) |
| 1 |
2 |
4 |
8 |
| Control |
DMSO |
0.74 ± 0.10 |
0.66 ± 0.10 |
0.54 ± 0.08 |
0.44 ± 0.05 |
| IND |
20 |
0.38 ± 0.08(49%) |
0.30 ± 0.09(55%) |
0.26 ± 0.08(52%) |
0.16 ± 0.05(64%) |
| Ethyl acetate fraction of T. conoides |
75 |
0.62 ± 0.07(16%) |
0.56 ± 0.10(15%) |
0.44 ± 0.05(19%) |
0.40 ± 0.06(9%) |
| 150 |
0.50 ± 0.09(32%) |
0.40 ± 0.09(39%) |
0.32 ± 0.07(41%) |
0.28 ± 0.10(36%) |
| 450 |
0.40 ± 0.06(46%) |
0.32 ± 0.08(52%) |
0.28 ± 0.07(48%) |
0.18 ± 0.08(59%) |
Values expressed in centimeters (mean ± standard deviation). Percentages indicated to the change in edema size with control group. Comparisons were made between control and drug treated groups, *P<0.05.
Table 1: Anti-inflammatory potential of ethyl acetate fraction of T. conoides on carrageenan induced paw edema in rat.
Anti-nociceptive of ethyl acetate fraction of T. conoides was performed in mice by following chemical and thermal methods. In chemical method, acetic acid induced abdominal writhes showed about 59.40 ± 1.86 in the control group over the period of 10 minutes (Figure 2). In treated groups, the number of abdominal writhes were significantly (P0<0.5) inhibited by standard drug, aspirin (13.80 ± 2.25) as well as ethyl acetate fraction of T. conoides (15.83 ± 0.86). Writhes inhibited by aspirin was about 77% over the control where as in ethyl acetate fraction of T. conoides was about 73.4% observed at the maximum dose 450 mg/kg bw. At the dose of 150 mg/kg bw, the writhes inhibition was about 44% over the control. In contrast, the writhes inhibition was found to be lower at the dose of 75 mg/kg bw (P0<0.5) which has shown about 17% over the control. The potential of ethyl acetate fraction of T. conoides on writhes was found to be significantly dose dependent.

Figure 2: Anti-analgesic potential of ethyl acetate fraction of T. conoides on acetic acid induced writhing in mice (C: control; Asp: aspirin; *P<0.05; Comparisons were made between control and drug treated groups).
The analgesic potential of ethyl acetate fraction of T. conoides was evaluated using tail immersion method in mice. The tail flick response was measured after administration of test and standard drugs at the time period of 1, 2 and 3 hrs. The pain response in the control group showed decrease reaction time 2.08 ± 0.18-1.60 ± 0.17 at time period of 1-3 hrs (Figure 3). Standard drug, aspirin significantly enhanced the reaction time in the range of 8.10 ± 0.33-11.00 ± 0.18 by reducing the pain response. The reduction of pain response seems to be better than control and ethyl acetate fraction treated groups. Among the ethyl acetate fraction tested dose, the highest enhanced reaction time was observed at 450 mg/kg bw (10.20 ± 0.37 at 3 h) and least at the dose 75 mg/kg bw (3.62 ± 0.24 at 3 h). At the dose of 150 mg/kg bw, the enhanced reaction time by reducing pain response observed to be 5.12 ± 0.45-8.12 ± 0.39 which was better than the control (P<0.05). Ethyl acetate fraction of T. conoides was significantly (P<0.05) reduce the pain response by the increase of reaction time with dose dependent manner.
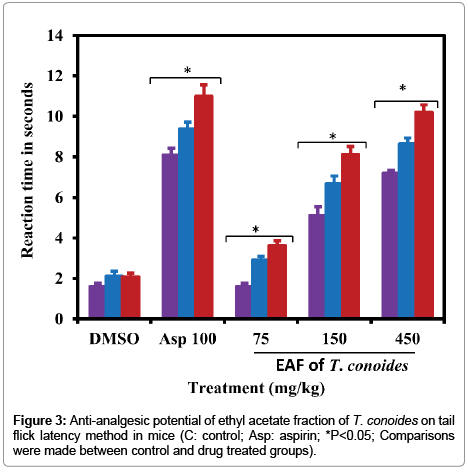
Figure 3: Anti-analgesic potential of ethyl acetate fraction of T. conoides on tail flick latency method in mice (C: control; Asp: aspirin; *P<0.05; Comparisons were made between control and drug treated groups).
Discussion
The antigenotoxicity of ethyl acetate fraction of T. conoides was evaluated against 4-NQO induced chromosome damage in mice bone marrow cells. Genotoxicity of 4-NQO occurred through its metabolite 4-hydroxyaminoquinoline 1-oxide (Ac-4-HAQO) which is readily interacts with DNA and forming adducts at the N2, C8 and of N6 position [15]. As consequence, the helical structure of the DNA changed and turns to micronuclei/chromosomal breakage [16]. In the present study, 4-NQO enhanced the frequency of MnPCEs by about 4.1 fold over the control group (16.0 MnPCEs/2500 PCEs; Figure 1). Pretreatment with ethyl acetate fraction of T. conoides effectively decreased the 4-NQO enhanced MnPCE frequency about 20-72% depending on dose tested (Figure 1). Moreover, the reduction of MnPCE by the highest dose of ethyl acetate fraction of T. conoides was well corresponded with their control value. The maximum reduction observed about 72% at dose 450 mg/kg bw which was 3.6-fold better than that of their lower dose, 75 mg/kg bw. In addition, at dose 150 mg/kg bw also showed 2.9 fold of activity over the dose at 75 mg/kg bw. The reduction of 4-NQO enhanced MnPCE frequency was significant (P<0.05) and dose dependent.
Under the damaged cell/tissue condition, several inflammatory mediators such as histamine, bradykinin, serotonine, and prostaglandins are released to stimulate the inflammation and nociceptors [16]. These mediators are occupied in tissues with high content of water and plasma during arachidonic acid metabolism via cyclo-oxygenase and lipo-oxygenase enzyme pathways [17]. The first phase of inflammation begins immediately up to an hour after injection of carrageenan by the release of histamine and serotonin whereas the second phase started after one hour and up to three hours by the release of bradykinin, protease and prostaglandins [18]. Anti-inflammatory effect of indomethacin and ethyl acetate fraction of T. conoides were showed significantly (P<0.05) better than that of control (Table 1). The highest dose of ethyl acetate fraction reduced the carrageenan induced paw volume about 59% which was more comparable to the standard drug (64%). The reduction of paw volume was found to be dose dependent. The acetic acid induced abdominal writhes in mice were significantly recovered from all the tested doses of T. conoides (Figure 2). Indomethacin and ethyl acetate fraction of T. conoides might be reduced inflammation through stabilizing the lysosomal membrane. Recent report on Turbinaria ornate extract revealed their better anti-inflammatory and free radical scavenging property due to fucoidan like sulfated polysaccharides [19]. The reduction of writhes by the highest dose of ethyl acetate fraction was 3.8 fold over the control and more comparable to the standard drug (13.80 ± 2.25). In addition, the analgesic effect of T. conoides on the tail immersion-test in mice was found to be dose dependent and significantly reduced the pain response by the increase of reaction time (Figure 3). The highest dose of T. conoides and standard drug were showed 5-4 fold pain reduction over the time periods when compared to the control value. In both writhes and tail immersion-test were found to be significantly (P<0.05) dose dependent. Turbinaria conoides reported to have better antipyretic activity by restoring many hematological and biochemical parameters under toxic environment [20]. It is well known that Turbinaria sp possessed potential antioxidant, anti-inflammatory and anticancer properties due to rich source bioactive compounds such as fucosterol, sulfated polysaccharides fucoidan, neutral glucan, guluronic and alginic acid [2,20].
Conclusion
Ethyl acetate fraction isolated from ethanol extract of T. conoides in order to understand the antigenotoxic and anti-inflammatory potential because it is being used as a traditional medicine. In-vitro antioxidant potential was performed and found to be highest in ethyl acetate fraction than that of hexane, dichloromethane, and aqueous fractions obtained from ethanol extract of T. conoides. Therefore, ethyl acetate fraction of T. conodes was used to evaluate the antigenotoxic and antiinflammatory potential and found to be significantly effective and dose dependent. The potential activity might be due to the presence of synergetic bio-active compounds like steroids, phenolics, flavonoids, reducing sugars, fucosterol, sulfated polysaccharides including fucoidan, neutral glucan, guluronic and alginic acid.
Acknowledgements
This contributed research work was supported by University Grant Commission (UGC), Government of India, New Delhi (TAM -8496).
Conflict of Interest
There was no conflict of interest.
17936
References
- Vatan O, Celikler S, Yildiz G (2011)In vitro antigenotoxic and anti-oxidative capacity of Hypnea musciformis (Wulfen) Lamouroux extract in human lymphocytes. Afr J Biotechnol 10: 484-490.
- Ananthi S, Gayathri V, Veeresh Kumar S, Meenakshi B, Vasanthi HR (2016) Attenuation of inammation by marine algae Turbinaria ornata in cotton pellet induced Granuloma mediated by fucoidan like sulphated polysaccharide. Carbohydrate Polymers 151: 1261-1268.
- Lee JC, Hou MF, Huang HW, Chang FR, Yeh CC (2013) Tang JY Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Inter 13:55.
- Shanmugam SK, Kumar Y, Sardar Yar KM, Gupta V, De Clercq E (2010) Antimicrobial and Cytotoxic Activities of Turbinaria conoides (J.Agardh) Kuetz.Iranian J Pharmaceut Res 9:411-416.
- Nabanita C, Ghosh T, Sinha S, Kausik C, Karmakar P, et al. (2010) Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chem 118:823-829.
- Rajeshkumar S, Malarkodi C, Gnanajobitha G, Paulkumar K, Vanaja M, et al. (2013) Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J Nanostructure Chem 3:44.
- Fitton JH, Stringer DN, Karpiniec SS (2015) Therapies from fucoidan: an update. Mar Drugs13:5920-5946.
- Chakraborty K, Praveen NK, Vijayan KK, Syda Rao G (2013) Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar. Asian Pac J Trop Biomed 3:8-16.
- Hu X, Jiang X, Hwang H, Liu S, Guan H (2004) Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur J Phycol39:67-71.
- Arumugam P, Ramamurthy P, Santhiya ST, Ramesh A (2006) Antioxidant activity measured in different solvent fractions obtained from Mentha spicata linn.: ananalysis by ABTS+ decolorization assay. Asia Pacific J Clin Nutr 15:20-24.
- Arumugam, P, Ramesh A (2009) Protective effects of solvent fractions of Mentha spicata (L.) leaves: evaluated on 4-nitroquinoline-1-oxide induced chromosome damage and apoptosis in mice bone marrow cells. Genet Mol Biol 32:847-852.
- Arumugam P, Gayatri Priya N, Subathra M, Ramesh A (2008) Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ Toxicol Pharmacol 26:92-95.
- Ferreira J, Floriani AEO, Cechinel-Filho V, Delle-Monache F, Yunes RA, et al. (2000)Antinociceptive properties of the methanolic extract and two triterpenes isolated from Epidendrum mosenii stems (Orchidaceae). Life Sci 66:791-802.
- Kumar JP, Shankar NB (2009) Analgesic activity of mollugo pentaphylla Linn. by tail immersion method. Asian J Pharmaceu Clin Res2:61-63.
- Kanojia D, Vaidya MM (2006) 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol 49:3056-3059.
- Hajhashemi V, Ghannadi A, Hajiloo M (2010) Analgesic and anti-inflammatory effects of Rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J Pharmaceu Res 9:163-168.
- Moura ACA, Silva ELF, Fraga MCA,Wanderley AG, Afiatpour P (2005) Antiinflammatory and chronic toxicity study of the leaves of Ageratum conyzoides L. in rats. Phytomedicine 12: 138-142.
- Gupta V, Kumar P, Bansal P, Singh R (2009) Anti-inflammatory and Anti-nociceptive Activity of Mitragyna parvifolia. Asian J Med Sci 1:97-99.
- Ananthi S, Gayathri V, Chandronitha C, Lakshmisundaram R, Vasanthi HR (2011) Fee radical scancenging and anti-inflammatory potential of a mairne borwn alga Turbinaria ornate (Turner) J. Agardh. IJMS 40: 664-670.
- Sadish Kumar S, Kumar V, Khan MSY, Anbu J, Sam KG (2009) Acute toxicity study and antipyretic effect of the brown alga turbinaria conoides (J. Agardh) Kuetz. AJTCAM 6: 233-240.









