Jamshid Farahati1,2*, Elana Gilman1, James Nagarajah2, Zohre Mousavi3, Rema Markous1 and Rasoul S Zakavi4
1Clinic for Nuclear Medicine, Bethesda, Duisburg, Germany
2Clinic and Policlinic for Nuclear Medicine, University Hospital Essen, Germany
3Endocrine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
4Nuclear Medicine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
*Corresponding Author:
Jamshid Farahati
Associate Proffessor, Clinic for Nuclear Medicine, Bethesda, Duisburg, Germany, Dorsten, Heerstr 219, 47053 Duisbrg, Germany
Tel: +49-203-6008-1451
Fax: +49-203-6008-199
E-mail: j.farahati@bethesda.de
Received date: July 29, 2016; Accepted date: August 19, 2016; Published date: August 22, 2016
Citation: Farahati J, Gilman E, Nagarajah J, et al. Increasing of Iodine Intake Decrease the High Risk of Goitre in Smoking Men. Transl Biomed. 2016, 7:3. doi: 10.21767/2172-0479.100085
Background: An association between smoking and the risk of goitre is reported in some, but not all studies. We investigated the association between smoking, goitre and iodine intake, with respect to different risk factors in 483 healthy men in Germany.
Methods: A total of 483 healthy male employees of 4 institutions in Essen in Western part of Germany, aged between 17 and 68 years (43.1 ± 9.6 y) were examined by ultrasound of the neck to determine the thyroid volume between April 2001 and April 2002. Information on age, BMI, smoking, daily use of iodized salt, and familial history of thyroid disorders were assessed by standardized questionnaires. Logistic regression analysis was performed to adjust for smoking, age, iodine status and familial history of goitre.
Results: The overall prevalence of goitre was 25.7%. Median thyroid volume among smokers, (23.4 ± 10.1 ml), was higher (p<0.001) as compared with non-smokers (19.4 ± 7.8 ml). Goitre was present in 77 out of 204 smokers (37.7%) vs. 47/279 (16.8%) in non-smokers (odds ratio: 3.0; p<0.001).
Smoking (p<0.001) and increasing age (p<0.001) was associated with goitre prevalence, but not BMI and the family history of goitre. Iodine status alone failed just to reach the significance level but revealed a clear trend. And there was significant prevalence of goitre in smoking men who did not take iodine salt compared to smoking men with regular intake of iodine salt (p=0.018).
Conclusions: Smoking increase the risk of goitre in men that can be decreased by taking iodine.
Keywords
Thyroid; Goiter; Iodine; Smoking; Male
Introduction
Goitre is a major health problem in Germany with an annual cost for treatment estimated at 1 billion Euro [1]. The etiology of simple endemic or sporadic goitre depends on genetic and environmental factors. The major factor, however, remain iodine deficiency [1,2].
Despite recent improvement of iodine supplementation in Germany since 1989 [3-8], the prevalence of goitre in Germany remains substantial [9,10].
An association between smoking and the risk of goitre have been reported in several studies predominantly or exclusively including females [11-17], but none in males.
In this prospective study, we investigated the prevalence of simple goitre among healthy smoker males in west part of Germany as compared to non-smokers with respect to age, iodine status and history of goitre in the first degree relatives.
Methods
Study design
A total of 483 healthy male employees of 4 institutions in Essen (Bethesda Essen and Karstadt) and Muelheim (Siemens and Mannesmann) in western part of Germany aged between 17 and 68 years (range: 43.1 ± 9.6 y) were examined by ultrasound of the neck to determine the thyroid volume.
Ultrasound was performed by one experienced investigator (J.F.) with a Siemens Sonoline SI-400 using a 7.5 MHz linear scanner. The ultrasound was performed according to standardized criteria and the volume of thyroid was determined as the sum of p/6 X lengths X width X depth of both lobes. Goitre was defined as a thyroid volume exceeding 25 ml.
All subjects with known thyroid disorder were excluded from this analysis. Information on date of birth, date of screening, sex, daily use of iodized salt, history of previous diseases including benign diseases of thyroid, history of thyroid disorders in the first degree relatives, and type and amount of smoking were assessed by standardized questionnaires.
Smoking was defined as consuming more than 10 cigarettes daily for at least 5 years. Because of small number of cases in groups of cigar and pipe smokers, we excluded 2 cigar and 2 pipe smokers from the analyses.
Statistics
The general descriptive analysis was performed using Microsoft Excel (Version 7.0) and PASW-Statistics (version 19.0). Values for continuous variables are given as means ±SD. Statistical differences between groups and comparison of frequencies between groups were calculated with the Chi- Square test or the Fisher’s exact test for the 2 × 2 contingency tables.
Linear regression analysis was performed for continuous variables and logistic regression analysis was performed for the binary dependent variable, presence of goitre, to adjust for age, smoking, iodine status and familial history of goitre. Parameters were estimated using a least square and maximum-likelihood methods and the significance level was assessed using t-Test or Wald test respectively. A p-value below 0.05 was considered to be statistically significant.
Results
The overall thyroid volume correlated (r=0.18; p<0.001) with increasing age (Figure 1). The overall prevalence of goitre was 25.7% (124/483). Median thyroid volume among smokers (23.4 ± 10.1 ml), was higher (p<0.001) as compared with nonsmokers (19.4 ± 8.0 ml).
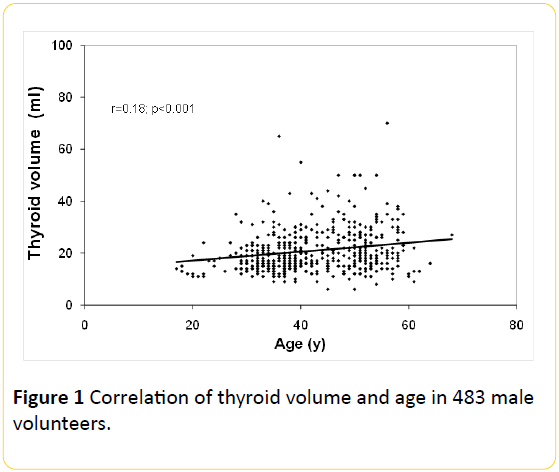
Figure 1: Correlation of thyroid volume and age in 483 male volunteers.
Goitre was present (Table 1 and Figure 2) in 77 out of 204 smokers (37.7%) vs. 47/279 (16.8%) in non-smokers (p<0.001) with an odds ratio of 3.0.
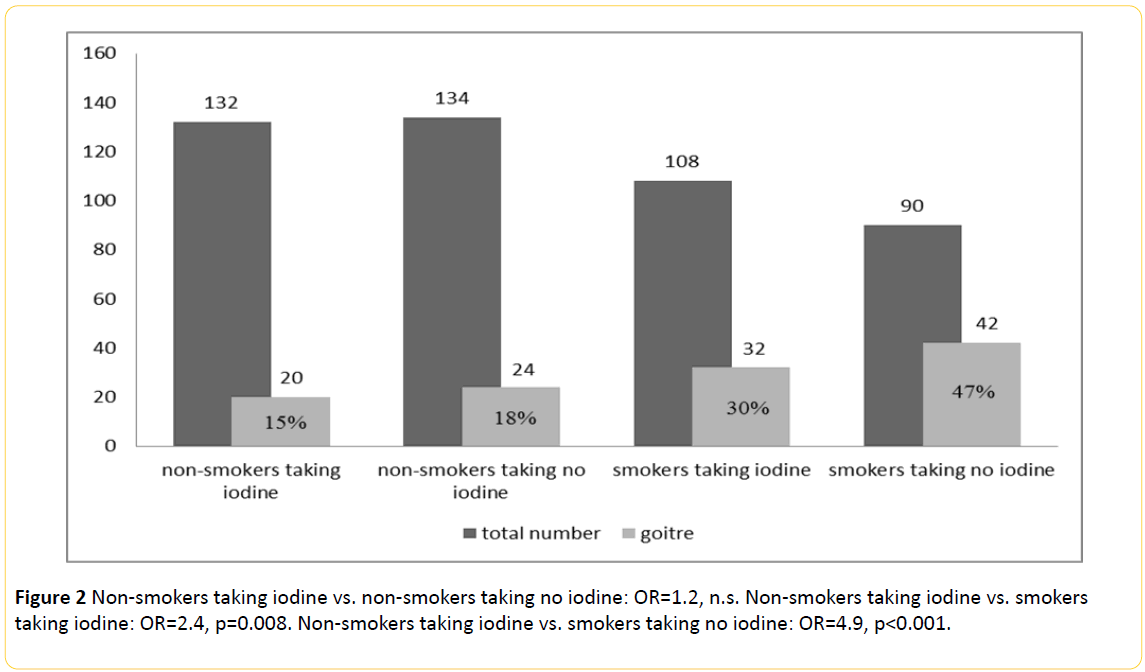
Figure 2: Non-smokers taking iodine vs. non-smokers taking no iodine: OR=1.2, n.s. Non-smokers taking iodine vs. smokers taking iodine: OR=2.4, p=0.008. Non-smokers taking iodine vs. smokers taking no iodine: OR=4.9, p<0.001.
| |
no goitre |
% |
goitre |
% |
OR (CI) |
p |
| N=483 |
359 |
74.3 |
124 |
25.7 |
|
|
| Age (mean) ± SD |
41.8 ± 9.8 |
|
46.6 ± 8.1 |
|
|
p<0.001 |
| BMI |
26.1 ± 4.0 |
|
26.7 ± 3.7 |
|
|
n.s. |
| Thyroid volume ± SD |
17.2 ± 3.9 |
|
33.0 ± 10.2 |
|
|
p<0.001 |
smokers
non-smokers |
127
232 |
62.3
83.2 |
77
47 |
37.7
16.8 |
3.0 (2.0;4.6) |
p<0.001 |
No children
children |
227
132 |
79.4
67.0 |
59
65 |
20.6
33.0 |
1.9 (1.3;2.9) |
p=0.003 |
No iodine salt
Iodine salt |
158
188 |
70.5
78.3 |
66
52 |
29.5
21.7 |
0.7 (0.4;1.0) |
p=0.056 (n.s.)
pone sided= 0.034 |
No family history
Family history |
294
65 |
74.2
74.7 |
102
22 |
25.8
25.3 |
1.0 (0.6;1.7) |
n.s. |
| Non-smokers taking no iodine |
110 |
82.1 |
24 |
17.9 |
|
poverall <0.001 |
| Non-smokers taking iodine |
112 |
84.8 |
20 |
15.2 |
0.8 (0.4;1.6) |
n.s. |
| Smokers taking no iodine |
48 |
53.3 |
42 |
46.7 |
4.9 (2.6;9.2) |
p<0.001 |
| Smokers taking iodine |
76 |
70.4 |
32 |
29.6 |
2.4 (1.3;4.4) |
p=0.008 |
| P<0.05 was considered as significant level. OR (CI): odds ratios with the range of confidence interval |
Table 1: Multivariate analyses of risk factors, age, BMI, smoking, iodized salt intake, family history of goiter on prevalence of goiter.
Smoking (<0.001) and increasing age (p<0.001) associated with goitre prevalence by the logistic regression analysis but not the history of goitre in the first degree relatives (Table 1) and the iodine status failed just to reach the significant level in this analysis (p=0.056).
Chi-square-Test shows a higher prevalence of goitre among smokers who don’t use iodine salt compared to smokers taking iodine regularly (29.6% vs. 46.7%, p=0.018, poverall<0.001). Among the non-smoking individuals the frequency of goitre stays by 15.2% with iodine intake and by 17.9% without iodine (n.s.).
Discussion
This study indicates smoking as the major risk factor for goitre in men. The median volume of thyroid in smokers was in general higher as compared to non-smokers. The risk of developing goitre in smoking men was approximately 3 times higher as compared to non-smokers. In addition, the prevalence of goitre in smokers was significantly lower in case of iodine intake.
Earlier investigations suggest that cigarette smoking may increase thyroid hormone secretion [18]. The first clinical record of association between smoking and goitre prevalence was published 1984. In this study Christensen et al. [11] evaluated the prevalence of goitre by palpation in 441 middle aged women and found higher goitre prevalence among smokers (14.8%) as compared to ex-smokers (3.8%) and nonsmokers (9.4%).
In 219 randomly selected hospital employees, Hegedus et al. [12] found a higher prevalence of goitre among smokers (30%) as compared to non-smokers (3%).
Berghout et al. [13] found no association between thyroid volume and smoking and no correlation between thyroid volume and tobacco consumption in an iodine-sufficient area.
Ericsson et al. [14] report in an epidemiological study from Sweden including more than 4000 subjects, a higher goitre prevalence among smoking females (14.2%) as compared to non-smokers (8.9%) and ex-smokers (7.0%).
Petersen et al. [15] found no increased prevalence of thyroid disease or goitre in 1154 middle-aged and older Swedish women with respect to smoking (14.3% smokers vs. 13.4% non-smokers).
Georgiadis et al. [16] investigated 187 employees of a hospital and their relatives and found goitrogen effect of smoking only in individuals with a family history of goitre.
However, these studies mostly or exclusively performed in female population, few suffer from insufficient number of subjects or inappropriate multivariate analysis of risk factors.
Knudsen et al. [17] report in a recent multivariate analysis on 4649 randomly selected sample of females and males (2002) on association between smoking and increased goitre prevalence, which was more pronounced in the most iodine deficient area.
The effects of smoking on the thyroid have been attributed to thiocyanate. Body fluid of smokers contains increased concentration of thiocyanate [19-22]. Thiocyanate inhibit iodide uptake and thyroid hormone synthesis and increase the efflux of iodide from the thyroid gland [22].
Indeed, recent improvement of iodine supplementation since 1989 reduced the prevalence of goitre especially in younger population [3-8], however, the prevalence of goitre in Germany remains substantial [9,10].
We conclude that smoking is an independent risk factor affecting the goitre prevalence in males. In addition, our study reveals that iodine intake can reduce the risk of goitre in smoker males significantly.
Acknowledgement
We thank Dr. Eberhard Heissen, Mrs. Vo and Mrs. Walschus for preparing the manuscript.
13705
References
- Kahaly GJ, Dietlein M (2002) Cost estimation of thyroid disorders in Germany. Thyroid 12: 909-914.
- Farahati J, Geling M, Mader U, Mortl M, Luster M, et al. (2004) Changing trends of incidence and prognosis of thyroid carcinoma in “Lower Frankonia”, Germany from 1981-1995. Thyorid.
- Hampel R, Gordalla A, Zollner H, Klinke D, Demuth M (2000) Continuous rise of urinary iodine excretion and drop in thyroid gland size among adolescents in Mecklenburg-West-Pomerania from 1993 to 1997. Exp Clin Endocrinol Diabetes 108: 197-201.
- Hampel R, Beyersdorf-Radeck B, Below H, Demuth M, Seelig K (2001) Urinary iodine levels within normal range in German school-age children. Med Klin 96: 125-128.
- Meng W, Schindler A, Horack S, Lux E, Muche A (1998) Renal iodine excretion by students in East Germany. A prospective study 1989 to 1996. Med Klin 93: 347-351.
- Gartner R, Manz F, Grossklaus R (2001) Representative data of iodine intake and urinary excretion in Germany. Exp Clin Endocrinol Diabetes 109: 2-7.
- Rendl J, Juhran N, Reiners C (2001) Thyroid volume and urinary iodine in German school children. Exp Clin Endocrinol Diabetes 109: 8-12.
- Manz F, Bohmer T, Gartner R, Grossklaus R, Klett M, et al. (2002) Quantification of iodine supply: representative data on intake and urinary excretion of iodine from the German population in 1996. Ann Nutr Metab 46: 128-138.
- Volzke H, Ludemann J, Robinson DM, Spieker KW, Schwahn C, et al. (2003) The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid 13: 803-810.
- Gartner R, Bechtner G, Rafferzeder M, Greil W (1997) Comparison of urinary iodine excretion and thyroid volume in students with or without constant iodized salt intake. Exp Clin Endocrinol Diabetes 105: 443-45.
- Christensen SB, Ericsson UB, Janzon L, Tibblin S, Melander A (1984) Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. J Clin Endocrinol Metab 58: 615-618.
- Hegedus L, Karstrup S, Veiergang D, Jacobsen B, Skovsted L, et al. (1985) High frequency of goitre in cigarette smokers. Clin Endocrinol 22: 287-292.
- Berghout A, Wiersinga WM, Smits NJ, Touber JL (1987) Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol 26: 273-280.
- Ericsson UB, Lindgarde F (1991) Effects of cigarette smoking on thyroid function and the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. J Intern Med 229: 67–71.
- Petersen K, Lindstedt G, Lundberg PA, Bengtsson C, Lapidus L, et al. (1991) Thyroid disease in middle-aged and elderly Swedish women: thyroid-related hormones, thyroid dysfunction and goitre in relation to age and smoking. J Intern Med 229: 407-413.
- Georgiadis E, Papapostolou C, Korakis T, Evagelopoulou K, Mantzoros C, et al. (1997) The influence of smoking habits on thyroid gland volume: an ultrasonic approach. J R Soc Health 117: 355-358.
- Knudsen N, Bulow I, Laurberg P, Perrild H, Ovesen L, et al. (2002) High occurrence of thyroid multinodularity and low occurrence of subclinical hypothyroidism among tobacco smokers in a large population study. J Endocrinol 175: 571-576.
- Melander A, Nordenskjold E, Lundh B, Thorell J (1981) Influence of smoking on thyroid activity. Acta Med Scand 209: 41-43.
- Bertelsen JB, Hegedus L (1994) Cigarette smoking and the thyroid. Thyroid 4: 327–331.
- Bartalena L, Bogazzi F, Tanda ML, Manetti L, Dell’Unto E, et al. (1995) Cigarette smoking and the thyroid. Euro J Endocrinol 133: 507–512.
- Bartalena L, Martino E, Marcocci C, Bogazzi F, Panicucci M, et al. (1989) More on smoking habits and Graves’ ophthalmo-pathy. J Endcrinol Invest 12: 733–737.
- Fukayama H, Nasu M, Murakami S, Sugawara M (1992) Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent anti-thyroid agent. Acta Endocrinologica 127: 520–525.







