Keywords
Type 2 diabetes, deterioration of glycemic control, glimepiride, rosiglitazone, monotherapy, antidiabetic drugs
Introduction
Type 2 diabetes mellitus is a progressive disease with deterioration of glycemic control over time. The increasing incidence of type 2 diabetes, in virtually all decades of human life, results, in the long run in an huge burden in health care cost and an endless series of complications. The nursing community, well aware, of the above has a significant role in the care setting of diabetic patients, whether in nursing homes or in the community [1] . As it was shown in the UKPDS monotherapeutic attempts often fail in the long run. The need to use drugs with different and complementary mechanisms of action frequently arises in daily nursing practice [2,3] .
Some patients do not accept insulin treatment because of the fear of needles and injections, the fear that the complications of diabetes are caused by insulin, and other false beliefs, and are willing to take as many antidiabetic pills to doctor is prepared to prescribe. The combination of an insulin secreting agent (usually a sulfonylurea) with metformin is commonly used in clinical practice. But when this potent combination is no longer able to provide acceptable glycemic control, the addition of an antidiabetic drug with a different mode action may lead to improved metabolic control [4,5] .
The insulin-sensitizing thiazolidinediones (TZD’s) are selective ligands of the nuclear transcription factor peroxisome-proliferator activated receptor γ (PPARγ) [6] .
PPARγ is expressed most abundantly in adipose tissue but is also found in pancreatic beta-cells, vascular endothelium and macrophages [7,8] .
The discovery of PPARγ as the target for TZD’s was followed by large-scale clinical trials of several agents. In January 1997, the first thiazolidinedione, troglitazone was approved as a glucose lowering therapy for patients in the United States with type 2 diabetes. Troglitazone was subsequently withdrawn from the market, in March 2000, because of hepatotoxicity. The two currently available PPARγ agonists, rosiglitazone and pioglitazone are considered relatively free of hepatotoxicity. The idiosyncratic liver toxicity observed with troglitazone does not seem to be a class effect of thiazolidinediones [9-11] .
Many physicians in everyday practice choose the monotherapeutic approach in treating type 2 diabetic patients. That is, they give an insulin-secreting agent as a first choice, usually a sulfonylurea. When the patient is no longer in control the usual practice is to increase the sulphonylurea dose in an effort to reduce hyperglycemia and control the disease [12] .
Glimepiride is the most potent sulphonylurea on a per-milligram basis and appears to be as effective as other sulphonylureas in reducing glucose levels when administered by 1-8 mg daily [13] .
In the current study we examined the effect of adding rosiglitazone to glimepiride treatment on glycemic control in patients with type 2 diabetes mellitus when compared to uptitrating the glimepiride dose.
Material and Methods
A total of 286 Greek type 2 diabetic patients, inadequately controlled, were enrolled in the study. All patients, in the beginning of the study were receiving only glimepiride 4 mg daily. Patients were randomized to 2 groups.
In the first group (n=146) the patients were given rosiglitazone 4mg b.d. plus glimepiride 4mg daily.
In the second group (n=140) the dose of glimepiride was uptitrated to a maximum of 8 mg per day. The characteristics of the patients were similar between the two groups as is shown in table 1.
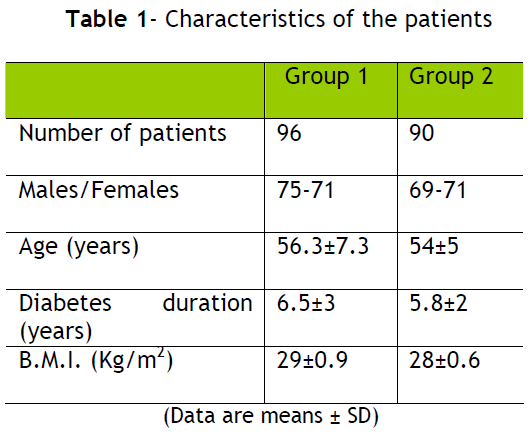
In the first group of patients there were 75 men and 71 women, the mean age was 56.3±7.3 years (mean±SD), diabetes duration was 6.5±3 years and B.M.I. was 29±0.9 kg/m2.
In second group of patients there were 69 men and 71 women, the mean age was 54±5 years, diabetes duration was 5.8±2 years and the B.M.I. was 28±0.6 kg/m2.
HbA1C was measured by high performance liquid chromatography.
All patients were given instructions for dieting and daily exercise by a community nurse with experience in diabetes. Especially the need for increasing the dose of glimepiride or adding second antidiabetic pill was explained to the patients in order to attain better glycemic control.
The Statistical analysis was performed with the method of t-test and p<0,05 was considered significant.
Results
Twenty-four weeks after the treatment modification there was a statistically significant decrease in HbA1C in the first group of patients but not in the second one. (table 2).
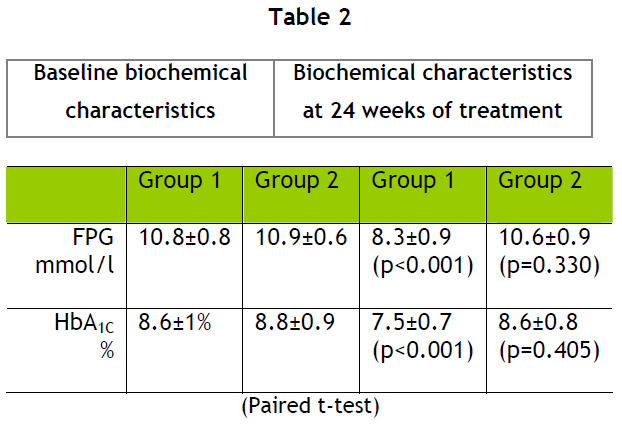
In the first group of patients the average baseline HbA1C before the treatment modification was 8.6±1% and baseline fasting plasma glucose (FPG) was 10.8±0.8 mmol/L.
After the treatment modification HbA1C was 7.5±0.7% (p<0.001) and FPG was 8.3±0.9 mmol/L (p<0.001).
In the second group of patients the average baseline HbA1C was 8.8±0.9% (p<0.001) and FPG was 10.9±0.6 mmol/L (p<0.001).
After the treatment modification HbA1C was 8.6±0.8% (p=0.405) and FPG was 10.6±0.9 mmol/L (p=0.330).
The combination of rosiglitazone and glimepiride produced significantly greater decreases in HbA1C and FPG than uptitrating glimepiride (p<0.001).
Side effects Hypoglycemic episodes were more frequent in the rosiglitazone plus glimeride group (14% vs 3%, p<0.01), body weight also increased more (3±0.5±0.2 kg, vs 1.1±0.2 kg, p<0.05) and oedema was more frequent in the first group of patients (14% vs 4%, p<0.001).
No change in liver function tests was noted in the patients in our treatment groups for the 24-week period of follow-up.
Discussion
Our findings are in accordance with those of other investigators who found that in inadequately controlled type-2 diabetic patients on treatment with a sulphonylurea, the addition of rosiglitazone produces significant improvement in glycemic control than increasing the dose of sulphonylurea [14] .
We have previously shown that in inadequately controlled type 2 diabetic patients, on treatment with a sulphonylurea and metformin the addition of rosiglitazone produces significant improvement in glycemic control and is safe and well tolerated [15] .
The adverse effects observed in our patients are those usually observed with these antidiabetic agents. The increase in body weight, with the use of thiazolidinediones, has been attributed to expansion of the subcutaneous fat depot, and in some patients to edema, whereas the mass of visceral fat remains unchanged [16] or decreases [17] .
No change in liver function tests was observed in our patients for the 24-week period of follow-up.
Rosiglitazone has only rarely been associated with severe liver reactions [18,19] .
Due the progressive nature of type – 2 diabetes mellitus and the decline in β-cell function monotherapy eventually fails to provide adequate glucose control in the long term, and combination therapy is frequently necessary.
Diabetes is recognized late in the disease process. Insulin resistance and β-cell function are both invariably present once type 2 diabetes is clinically apparent. Our therapies are usually oriented to the end of the process, namely blood glucose. It is rational that therapy should be oriented toward the dual pathophysiology of the diabetic process and not just the dysglycemic end of it. Addressing the relative insulin deficiency, with an insulin-secreting agent, and trying to overcome insulin-resistance by promoting more and more insulin secretion by the β –cell may well be the road to β –cell exchaustion. At the other hand addressing insulin resistance with an insulin-sensitizing agent, such a thiazolidinedione, may preserve β –cell function by rendering peripheral tissues more sensitive to insulin and thus achieving glycemic control with lower insulin levels. The combination of an insulin-secreting and an insulin-sensitizing agent seems most appropriate given the dual pathophysiology of the process leading to hyperglycemia. Earlier use of combination therapy, even as initial therapy will facilitate reaching goal and relieving glucotoxicity. [20,21]
Glucotoxicity likely plays some role in declining β –cell function and may not occur, to the same extent, with thiazolidinedione treatment.
As is shown, by the results of this study, it may be advantageous to begin combination therapy earlier in the course of the disease rather than increasing the dose of single current agent in order to achieve better glycemic control.
Patient education as to the need of using different and multiple drugs is essential. Diabetic patients are often treated with multiple drugs, given the frequent coexistence of hypertension, dyslipidemia, and cardiovascular disease. Thereby diabetic individuals may be unwilling to add more pills.
The nurse has a significant task in informing the patient that diabetes is a complex disease with dual pathophysiology. And the optional glucose control is to achieved only at the cost of adding antidiabetic pills which act synergistically. Moreover, nurses have an essential role in implementing health programs that focus positively not only on the importance of physical activity and health eating habits but on the patients compliance with the therapy prescribed, as well. Thus in diabetes mellitus, patient education is paramount and nurse as an educator has a central and extremely important role in it. [22]
No financial associations or competing interests exist between the authors and the companies of the drugs mentioned in the manuscript.
Conclusion
Given the dual pathophysiology of type 2 diabetes mellitus may be advantageous to begin combination treatment earlier in the course of the disease rather than increasing the dose of a single current agent in order to achieve better glycemic control. The combination of rosiglitazone and glimepiride produces significantly greater decrease in HbA1C, and consequently better glycemic control, than uptitrating glimepiride dose.
3474
References
- Haas L. Management of diabetes mellitus medications in the nursing home. Drug Aging 2005;22(3):209-18.
- Turner RC, Cull CA, Frighi V, Holman RR: Glycemic control with diet, sulfonylurea, metformin or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) : UK Prospective Diabetes Study (UKPDS) Group. JAMA 281: 2005- 2012, 1999.
- Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC: Sulphonylurea failure in non-insulin dependent diabetic patients over six years: UK Prospective Diabetes Study (UKPDS) Group. Diabet. Med. 15:297-303, 1998.
- Harmon C, Willoughby DF, Floyd C. A lesson in early morning hyperglycemia. Nurse Pract, 2004;29(11):58-63.
- Lehman JM, Moore LB, Smith?Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferators activated receptor gamma (PPAR gamma). J Biol Chem 1995;270(22):12953-6.
- Wilson TM, Lambert MH, Kliewer SA. Peroxisome proliferators-activated receptor gamma and metabolic disease. Ann Rev Biochem 2001;70:341-67.
- Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandelwalle B, Desreumaux P, et al. Expression of PPAR gamma in normal human pancreatic islet cells. Diabetologia 2000;43(9):1165-9.
- Quarry-Horn JL, Evans BJ, Kerrigan JR. Type 2 diabetes mellitus in youth. J Sch Nurs. 2003;19(4):195-203.
- Komajda M, McMurray JJ, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31(7): 824?831.
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes: a multicentre, randomised, open-label trial. Lancet 2009;373(9681):2125-2135.
- Drass JA, Peterson A. Type II diabetes: exploring treatment options. Am J Nurs 1996;96(11):45-9.
- Rosenstock J, Samols E, Muchmore DB, Schneider J. Glimeriride, a new once daily sulphonylurea. A double-blind placebo controlled study of NIDDM patients. Glimepiride Study Group. Diabetes Care. 1996;19(11): 1194-9.
- Kerenyi Z, Samer H, James RE: Improved glycemic control in individuals with type 2 diabetes when treated with rosiglitazone plus 7.5mg of glibenclamide compared to increasing the glibenclamide dose to 15mg. Diabetologia : 46, Suppl.2: Abstr. 834, 2003.
- Kiayias JA, Vlachou ED, Theodosopoulou E, Lakka-Papadodima E. Rosiglitazone in combination with glimepiride plus metformin in type 2 diabetic patients. Diabetes Care 2002;25(7):1251-52.
- Carey DG, Cowin GI, Galloway GJ, Jones NP, Richards JC, Biswas N, Doddrell DM. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients. Obes Res 2002;10(10):1008-15.
- Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, et al. Activators of PPARγ have depot-specific effects on human preadipocyte differentiation. J Clin Invest 1997;100(12):3149-53.
- Forman LM, Simmons BA, Diamond RH. Hepatic failure in a patient taking rosiglitazone. Ann Intern Med 2000;132(2):118-121.
- Gouda ME, Khan A, Swhartzi, Cohen R. Liver failure in a patient treated with long term rosiglitazone therapy. Am J Med 2001;111(7):584-85.
- Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rau E, Dailey G. Triple therapy in type 2 diabetes: Insuline glargine or rosiglitazone added to combination of sulpholylurea plus metformin in insulin-naive patients. Diabetes Care 2006;29(3):554-559.
- Lingway I, Legendre J, Kaloyanova P, Zhang S, Adams-Huet B, Raskin P. Insulin- Based Versus Triple oral therapy by newly diagnosed type 2 diabetes. Diabetes Care 2009;32(10):1789-1795.







