Meriç Lütfi Avsever1*, T. Tansel Tanr?kul2, Derya Güroy3, Seçil Metin4, Hasan Ak?it5, Serra Tunal?gil1
?zmir-Bornova Veterinary Control Institute, Fish Diseases National Referance Laboratory, Department of Bacteriology, Bornova, ?zmir- Turkey
University of Aegean, Faculty of Fisheries, Department of Fish Disease, Bornova, ?zmir- Turkey
University of Yalova, Department of Aquaculture, Armutlu Vocational College, Yalova- Turkey
University of Süleyman Demirel, Faculty of E?irdir Fisheries, Department of Fish Disease, Isparta- Turkey
University of Bal?kesir, Faculty of Veterinary Medicine, Department of Biochemistry, Bal?kesir-Turkey
Corresponding Author:
Meriç Lütfi AVSEVER
?zmir-Bornova Veterinary Control Institute
Fish Diseases National Referance Laboratory
Department of Bacteriology, TR-35100 Bornova, ?zmir-TURKEY
Tel: +90 232 388 00 10/ 145
Fax: + 90 232 388 50 52
E-mail: lutfiavsever@gmail.com
Received Date: 22.02.2013 Accepted Date: 01.10.2013 Published Date: 05.03.2014
Key words
Blood parameters, Lactococcus garvieae, Rainbow trout (Oncorhynchus mykiss Walbaum, 1792)
Anahtar Kelimeler
Gökku?a?? alabal??? (Oncorhynchus mykiss Walbaum, 1792), Kan parametreleri, Lactococcus garvieae.
Introduction
L. garvieae from Lactococcus genus of Strep-tococcaceae, is Gram positive non-motile lactic acid bacteria which produce α- haemolytic colo-nies in blood agar. They are non spore-forming, non-acid fast cocci which are catalase and oxida-se positive (Ravelo et al. 2003; Vendrell et al. 2006). Lactococcosis caused by L. garvieae is an infectious disease of cultured rainbow trout and outbreaks of this disease generally occur during summer months when water temperatures rise above 16°C (Facklam and Eliot, 1995). Non spe-cific symptoms of haemorrhaging and congestion are seen in L. garvieae infections (Kusuda et al. 1991; Domenech et al. 1996) and the others symptoms are immobility, darkeninig of skin and exophtalmia (Collins et al. 1984).
Conventional microbiological methods are of-ten used in L. garvieae identification (Austin and Austin, 2007; Koneman et al. 1997; Timur and Timur, 2003) but identification with these met-hods are inefficient and time consuming (Holt et al. 1994). Polymerase Chain Reaction (PCR) is reported to be an easier and faster identification method for this agent (Zlotkin et al. 1998).
Blood parameters of fish are affected by many factors, among which water quality and infecti-ous diseases rank first. They show significant changes in many septicemic bacterial and viral infections such as Motile Aeromonas Septicemia (MAS), Flavobacteriosis, Vibriosis, Infectious Haematopoietic Necrosis (IHN), Infectious Panc-reatic Necrosis (IPN) and Viral Hemorrhagic Septicemia (VHS). These changes in blood pa-rameters is due to losses such as haemorrhagia and the impact of the infectious disease on vital organs such as kidney, spleen, liver and pancreas (Austin and Austin, 2007; Vosyliene, 1996).
The aim of this work is to investigate the im-pact of Lactococcosis that naturally ocured in rainbow trout on certain blood parameters.
Materials and Methods
Sampling
The outbreak was seen in rainbow trout weighing 200-300 g. in a farm located in the Ae-gean region of Turkey in June, 2012. Ten dis-eased (D1-10) and 10 clinically asymptomatic fish (C1-10) were used in this work. For blood sampling, the fish were anesthetized with 2-phenoxyethanol (Sigma) at a concentration of 0.30 ml L-1 and blood from tail fins of diseased and control fish were was drawn asceptically into containers with EDTA.
Isolation and identification of bacteria
Liver, spleen and kidney samples of fish were inoculated on Trypticase-soy agar (TSA, LABM), Blood Agar (LABM) and incubations in 25°C for 48 hours were carried out. After incuba-tion, colonies were purified and identified according to their physiological, biochemical and enzymatic characteristics (Holt et al. 1994).
Genotypic confirmation of isolates
Isolates were confirmed by PCR (Zlotkin et al. 1998). DNA Extraction was carried out with boiling method (Çiftçi et al. 2009) According to this method colonies grown in TSA were sus-pended in DPEC-treated water (DNase-RNase free) and and was boiled for 10 min. in 100 oC. This was followed by a centrifuge step in 10.000 rpm for 10 minutes. Supernatan was discarded and remains in the tube were used as the template DNA. In PCR amplification pLG-1 (5’-CATAACAATGAGAATCGC-3’) ve pLG-2 (5’-GCACCCTCGCGGGTTG-3’) oligonucleotide primers were used. DEPC-treated water, 1XPCR Buffer, 1.5 Mm MgCl2, 0.2 Mm of each d NTP, 1.0 U Taq polymerase, 1 μM of each primer and 5 μl template DNA was used in the PCR master-mix. The amplification consisted of 35 cycles and the steps were an initial denaturation in 94°C, followed by a 1 min. denaturation in 94°C, 1 min of annealing in 55°C, an extension of 1.5 min. in 72°C and 1.5 min.of final extension in 72°C. As a result, 1100 bp long amplification product was considered to be positive. Amplification products were visualized on a 1.5% agarose gel and 100 bp. DNA marker was used. Positive and negative controls were Lactococcus garvieae ATCC 43921 and Enterecoccus faecalis ATCC 29212, respectively.
Investigation of blood parameters
After aenesthesia, blood from tail fins of dis-eased and control fish were was drawn ascepti-cally into containers with EDTA. Blood samples were analyzed for with a blood count device cali-brated for fish blood (Mindray BC 2800, Tur-key). White Blood Cell, (WBC), Red Blood Cell, (RBC), Haemoglobin (Hb), Platelet Total Value, (PLT), Mean Platelet Volume, (MPV) and Plate-let Distribution Width (PDW) values were re-searched.
Statistical analyses of blood parameters
For the statistical analyses of data; SPSS (for Windows Release 11.5 Standart Versiyon Copy-right © Spss Inc. 1989-2001) was used. With an independant sampling test, data on blood parame-ters in these two groups of fish were compared. The P<0.05 were accepted significant.
Results and Discussion
Clinical symptoms for Lactoccosis in fish were haemorrhages in different parts of the body, darkening of skin and exophtalmia in some (Figure 1). In necropsy, anemia in liver and spleno-megaly was observed. Gram stained slides pre-pared from internal organs of infected fish re-vealed Gram positive bacterial colonization (Figure 2) whereas similiar findings were not ob-served in control group fish.
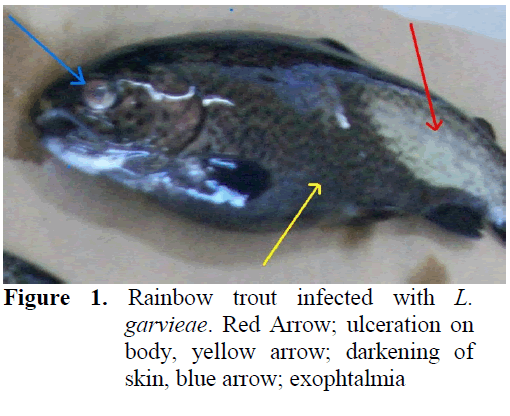
Figure 1: Rainbow trout infected with L. garvieae. Red Arrow; ulceration on body, yellow arrow; darkening of skin, blue arrow; exophtalmia
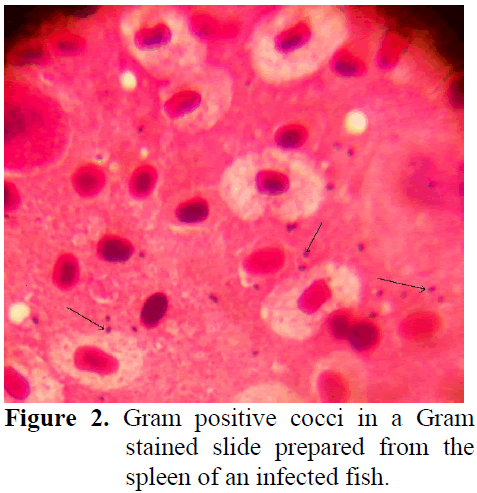
Figure 2: Gram positive cocci in a Gram stained slide prepared from the spleen of an infected fish.
Although L. garviae was isolated from all dis-eased fish, no bacterial pathogens were detected in asymptomatic fish. No differences were ob-served in phenotypical characterization of iso-lates and results are summarized (Table 1). Af-terwards, PCR confirmation was carried out (Figure 3).
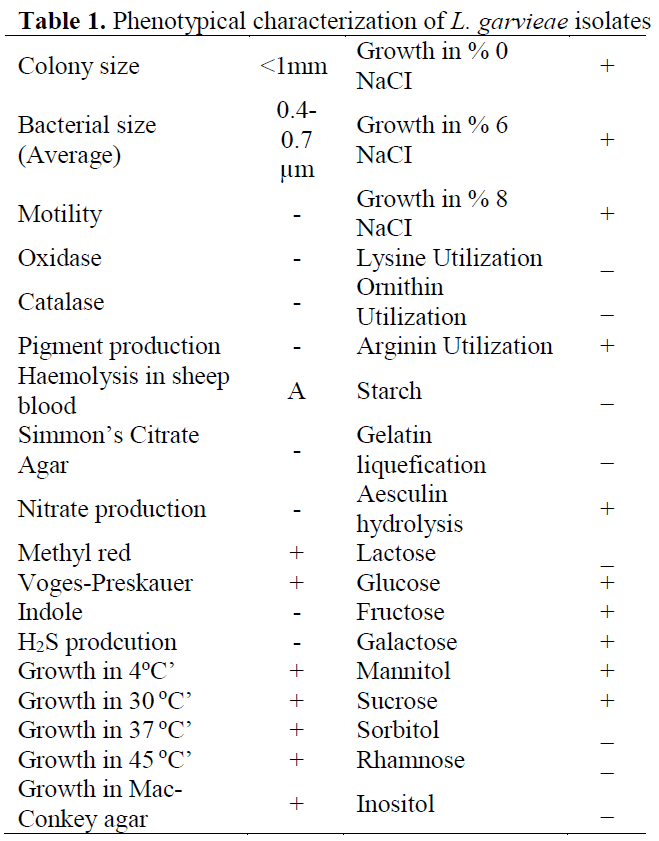
Table 1: Phenotypical characterization of L. garvieae isolates
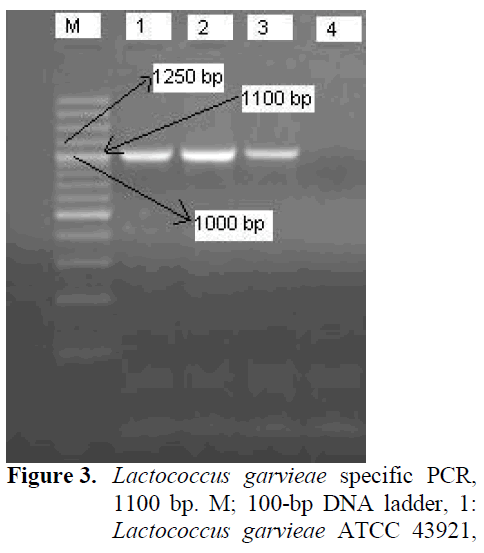
Figure 3: Lactococcus garvieae specific PCR, 1100 bp. M; 100-bp DNA ladder, 1: Lactococcus garvieae ATCC 43921, 2-3: isolates. 4: Enterecoccus faecalis ATCC 29212
Statistical analyses of WBC, RBC, HGB, PLT, MPV ve PDW values obtained from L. arvieae infected and control fish (Table 2).
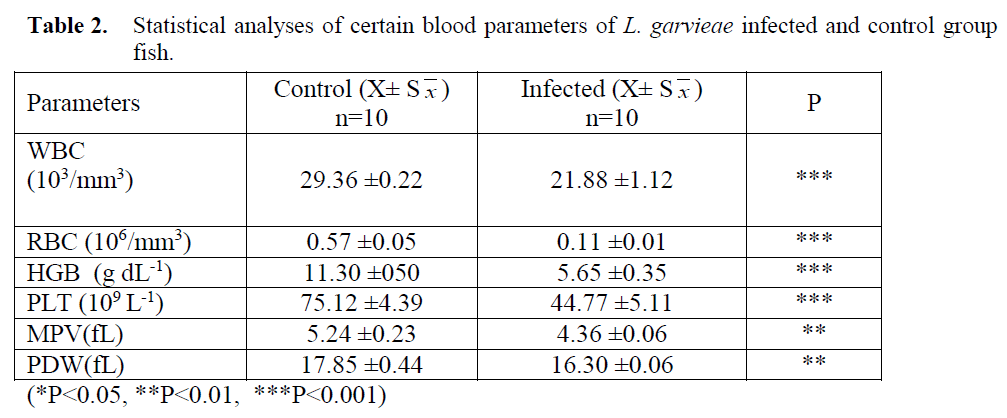
(*P<0.05, **P<0.01, ***P<0.001)
- Table 2: Statistical analyses of certain blood parameters of L. garvieae infected and control group fish.
Veterinary haematology is more common in small animal practice; research on blood parame-ters in fish is limited (Dethlof et al. 1999) and analyses are generally carried out manually (Vo-syliene, 1996; Cak?c? and Ayd?n, 2006; Handy et al. 1999; Zorriehzahra et al. 2010). As fish erythrocytes have nuclei, standard blood count devices record them as leukocytes. Thus, for fast and reliable results with automated devices; the system has to be calibrated with standard (fish) blood. Besides, blood sampling in fish requires special skills as veins are invisible and hard to manipulate. As a result of all these hindrances; determining blood parameters of fish haematolo-gy is harder than other animals.
Investigating haematological parameters is an effective way in determining the health status of fish (Blaxhall, 1972; Rehulka, 2002; Martins et al. 2008). Erythrocyte (RBC) count in fish natu-rally infected with L. garvieae was found to be significantly lower than the control group. Simi-lar findings of lower RBC values were also re-ported in coho salmon (Oncorhynchus kisutch) infected with V. anguillarum, in rainbow trout infected with Aeromonas sobria, A. caviae, Aer-omonas/Streptococcus, Y. ruckeri and V. anguil-larum, in tilapia infected with Streptoccus iniae, in carp infected with A. hydrophila and also in Asian chyclid fish (Etroplus suratensis) with epi-zootic ulcerative syndrome (Harbell et al. 1979; Barham et al. 1980; Altun and Diler, 1996; Pathiratne and Rajapakshe, 1998; Rehulka, 2002; Harikrishnan et al. 2003; Chen et al. 2004; Ceylan and Altun, 2010). In Nile tilapia experi-mentally infected with Enterococcus sp., RBC values were reported to be unchanged (Martins et al. 2008).
Rainbow trout infected with lactococcosis were also found to have lower WBC values (p<0.001). Although in early stages of experi-mentally induced Yersiniosis and Vibriosis, leu-kocyte counts were reported to rise, they were seen to decline as the disease progressed (Altun and Diler, 1996; Ceylan and Altun, 2010). How-ever, Martins et al. (2008), have observed in-creasing WBC values in Nile tilapia experimen-tally infected with Enterococcus sp.
Haemoglobin counts were reported to be sig-nificantly lower in rainbow trout infected with Y. ruckeri, Vibrio anguillarum, A. salmonicida, Aeromonas/Streptococcus, in Atlantic salmon with cold water Vibriosis, in coho salmon (On-corhynchus kisutch) with V. anguillarum and in Asian Chyclid fish (Etroplus suratensis) with ep-izootic ulcerative syndrome (Harbell et al. 1979; Barham et al. 1980; Altun and Diler, 1999; Pathiratne and Rajapakshe, 1998; Ceylan and Al-tun, 2010). HGB values obtained in this work were seen to be lower in rainbow trout due to lac-tococcosis (p<0.001).
Different researchers have noted important in-crease in platelet counts in experimentally infect-ed with Y. ruckeri, V. anguillarum, Aeromonas salmonicida and Renibacterium salmoninarum and in Nile tilapia infected with Enterococcus sp (Bruno and Munro, 1986; Demirdö?en, 1997; Al-tun and Diler, 1999; Ceylan and Altun, 2010; Martins et al. 2008).. In this research, Lactococ-cosis infection in rainbow trout was found to lead to a decrease in platelet count
Conclusion
Values for White Blood Cell, (WBC), Red Blood Cell, (RBC), Haemoglobin (Hb), Platelet Total Value, (PLT), Mean Platelet Volume, (MPV) and Platelet Distribution Width (PDW) in rainbow trout naturally infected with Lacto-coccus garviae were found to decrease by Con-trol group (p<0.01, p<0.001).
257
References
- Altun, S., Diler, Ö., (1999). Yersinia ruckeri ile infekte edilmis gökkusag? alabal?klar?nda (Oncorhynchus mykiss) Hematolojik ?ncelemeler, Turkish Journal of Veterinary and Animal Sicences, 23: 301-309
- nAustin, B., Austin, D.A., (2007) Bacterial Fish Pathogens: Disease in Farmed and Wild Fish. 3th ed.(Revised), Praxis Publishing Ltd, Chichester, UK, 457pp
- nBarham, W.T., Smit, G.L., Schoonbee, H.J., (1980). The haematological assessment of bacterial infection in rainbow trout, Salmo gairdneri Richardson, Journal of Fish Biol-ogy, 17(3): 275-281
- nBlaxhall, P.C., (1972). The haematological as-sessment of the health of freshwater fish. A review of selected literature, Journal of Fish Biology, 4(4): 593-604. doi: 10.1111/j.1095-8649.1972.tb05704.x
- nBruno, D.W., Munro, A.L.S., (1986). Haemato-logical assessment of rainbow trout, Salmo gairdneri Richardson, and Atlantic salmon, Salmo salar L., infected with Renibacterium salmoninarum, Journal of Fish Diseases, 9(3): 195-204. doi: 10.1111/j.1365-2761.1986.tb01004.x
- nCakici, H., Aydin S., (2006) Changes in Blood Parameters of Rainbow Trout (Oncorhyn-chus mykiss Walbaum) after Physical Pollu-tion, Journal of Applied Animal Research, 29(1): 77-80. doi: 10.1080/09712119.2006.9706576
- nCeylan, M., Altun, S.,(2010). Bibrio anguillarum ile infekte edilmi? gökkusa?? alabal?klar?nda (Oncorhynchus mykiss) Hematolojik ?ncelemeler, Uluda? University Journal of the Faculty of Veterinary Medicine, 29(2): 35-42
- nChen, C.Y., Wooster, G.A., Bowser, P.R., (2004). Comparative blood chemistry and histopathology of tilapia infected with Vib-rio vulnificus or Streptococcus iniae or ex-posed to carbon tetrachloride, gentamicin, or copper sulphate, Aquaculture, 239(1-4): 421-443. doi: 10.1016/j.aquaculture.2004.05.033
- nCollins, M.D., Farrow, F.A.E., Phillips, B.A., Kandler, O., (1984) Streptococcus garvieae sp nov. and Streptococcus plantarum sp. nov, Journal of General Microbiology, 129: 3427-3431
- nÇiftci, A., Findik, A., Onuk, A.E., Sava?an, S., (2009) Detection of Methicillin resistamce and slime factor as Staphylococcus aureus in bovine mastitis, Brazilian Journal of Micro-biology, 40: 254-261. doi: 10.1590/S1517-83822009000200009
- nDemirdö?en, N., (1997). Deneysel olarak Aer-omonas salmonicida ile infekte edilen gök-kusag? alabal?klar?nda (Oncorhynchus mykiss) baz? hematolojik parametreler, Yüksek Lisans Tezi, ?stanbul Üniversitesi Fen Bilimleri Enstitüsü, 54s
- nDethloff, G.M., Schlenk D, Khan S., Bailey, H.C., (1999) The Effects of Copper on Blood and Biochemical Parameters of Rain-bow Trout (Oncorhynchus mykiss), Archives of Environmental Contamination and Toxi-cology, 36: 415-423. doi: 10.1007/PL00006614
- nDomenech, A., Fernandez-Garayzabal, J.F., Pasqual, C., Garcia, J.A., Cutuli, M.T., Moreno, M.A., Collins, M.D., Dominguez. L., (1996) Streptococcosis in cultured turbot, Scophthalmus maximus (L.), associated with Streptococcus parauberis, Journal of Fish Diseases, 19: 33-38. doi: 10.1111/j.1365-2761.1996.tb00117.x
- nFacklam, R.R., Eliot, J.A., (1995). Identification, classification, and clinical relevance of cata-lase-negative, Gram-positive cocci, exclud-ing the streptococci and enterococci, Clini-cal Microbiology Reviews, 8: 479-495
- nFihman, V., Raskine, L., Barrou, Z., Kiffel, C., Riahi, J., Bercot, B., Sanson-Le Pors, M.J., (2006). Lactococcus garvieae endocarditis: identification by 16S rRNA and sodA se-quence analysis, Journal of Infection, 52: 3-6. doi: 10.1016/j.jinf.2005.04.021
- nHandy, R.D., Sims, D.W., Giles, A., Campbell, H.A., Musonda, M.M., (1996). Metabolic trade-off between locomotion and detoxifi-cation for maintenance of blood chemistry and growth parameters by rainbow trout (Oncorhynchus mykiss) during chronic die-tary exposure to copper, Aquatic Toxicology, 47: 23-41. doi: 10.1016/S0166-445X(99)00004-1
- nHarbell, S.C., Hodgins, H.O., Schiewe, M.H., (1979). Studies on the pathogenesis of vibri-osis in coho salmon Oncorhynchus kisutch (Walbaum), Journal of Fish Diseases, 2(5): 391-404. doi: 10.1111/j.1365-2761.1979.tb00391.x
- nHarikrishnan, R., Nisha Rani, M., Balasundaram, C., (2003). Hematological and biochemical parameters in common carp, Cyprinus car-pio, following herbal treatment for Aer-omonas hydrophila infection, Aquaculture, 221(1-4): 41-50. doi: 10.1016/S0044-8486(03)00023-1
- nHolt, J.G., Krieg, N.R., Sneath, P.H.A., Williams, S.T., (1994). Bergey’s manual of determina-tive bacteriology. 9th ed. Baltimore, Md: The Williams & Wilkins Co, pp. 527–558
- nKoneman, E.W., Allen, S.D., Janda, W.M., Schreckenber, P.C., Winn, W.C., (1997) Color Atlas of Diagnostic Microbiology, 5th ed., Lippincott- Raven Publishers, Philadel-phia, USA, pp.121-162
- nKusuda, K., Kawai, K., Salati, F., Banner, C.R., Fryer, J.L., (1991). Enterococcus seriolicida sp. nov, a fish pathogen, International Jour-nal of Systematic Bacteriology, 41: 406-409. doi: 10.1099/00207713-41-3-406
- nMartins, M.L, Mouriño, J.L.P., Amaral, G.V., Vieira, F.N., Dotta, G., Bezerra, A.J.M., Pedrotti, F.S., Jerônimo, G.T., Buglione-Neto, C.C., Pereira, G. Jr., (2008). Haemato-logical changes in Nile tilapia experimental-ly infected with Enterococcus sp., Brazilian Journal of Biology, 68: 631-637. doi: 10.1590/S1519-69842008000300025
- nPathiratne, A., Rajapakshe, W., (1998). Hemato-logical changes associated with epizootic ul-cerative syndrome in the Asian cichlid fish, Etroplus suratensis, Asian Fisheries Scien-ce, 11(3-4): 177-316
- nRavelo, C., Magarin˜os, B., Lo´ pez-Romalde, S., Toranzo, A.E., Romalde, J.L., (2003). Mole-cular fingerprinting of fish-pathogenic Lac-tococcus garvieae strains by random ampli-fied polymorphic DNA Analysis, Journal of Clinical Microbiology, 41: 751-756. doi: 10.1128/JCM.41.2.751-756.2003
- nRehukla, J., (2002). Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): clinical pathology, haematology and biochemistry, Acta veterinaria brno,71(3): 351-360. doi: 10.2754/avb200271030351
- nTimur, G., Timur, M., (2003). Bal?k hastal?klar?. ?stanbul Üniversitesi Su Ürünleri Fakültesi Yay?nlar? No: 5, Dilek Ofset Matbaas?, ?s-tanbul, 538s
- nVosyliene, M.Z., (1996). Hematological parame-ters of rainbow trout (Oncorhynchus mykiss) during short-term exposure to copper, Eko-logija/Ehkologiya/Ecology, 3: 12-18
- nZorriehzahra, M.J., Hassan, M.D., Gholizadeh, M., Saidi, A.A., (2010). Study of some he-matological and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fry in western part of Mazandaran province, Ira-nian Journal of Fisheries Sciences, 9(1): 185-198.











