Keywords
MDR bacteria; Ag-NPs; ZnO-NPs; Antibiotics-nanoparticles combination; Antibacterial activity
Introduction
Microbiologists and pharmacists are in continuous challenge to deal with, control and treat diseases caused by microbes we face daily. Recently the global public health is in a serious threat with the increasingly introduced antimicrobial resistance of the infecting pathogens to the ordinary known treatments. The antimicrobial resistance is defined as the resistance of a microorganism to an antimicrobial drug that was originally effective for the treatment of infections caused by it. Resistant microorganisms such as bacteria, fungi, viruses and parasites are able to withstand the attack by antimicrobials such as; antibacterial (antibiotics), antifungal, antiviral and antimalarial drugs, respectively [1]. As a result, those standard treatments become ineffective and infections persist with prolonged illness, higher health care expenditures and greater chance for death with the risk of spreading to the others [2]. It is important to mention that the antimicrobial resistance's development is present in either developing or advanced countries with impressive increase in the numbers of victims who died by infections caused by resistant microorganisms every year. Melake et al. [3] studied the prevalence of MDR bacterial isolates causing burn infections from 105 patients admitted the Burn Unit of Menoufia University Hospital. They reported that about 36% of patients had burn wound infections and about 63% of the bacterial growth was MDR isolates.
Although, resistance of bacteria to antibiotics could occur normally as a natural phenomenon, certain human actions accelerate its emergence and spread where; the inappropriate use of antibiotics either by overuse or misuse can lead to the evolution of new MDR bacteria. If these trends persist and resistance continues to rise, some reports estimated that by 2050 there will be ten million antimicrobial resistance related deaths worldwide, costing the world up to 100 trillion $ (2). Unfortunately, the discovering of new antibiotics is considered a real challenge to the researchers and pharmaceutical companies due to the difficulty of identifying novel bacterial targets that could be used for the innovation of new classes of safe and effective antimicrobial agents by a rate covering the incredible increase in bacterial resistance among the world [4].
Nowadays, there is a great interest in integrating nanotechnology with biology in order to develop new antimicrobial drugs based on nanotechnology with higher affectivity. It was reported that some noble metals like gold and silver exhibit interesting antibacterial activity [5-7]. However, only few studies have compared the efficacy of noble metals nanoparticles such as silver nanoparticles (Ag-NPs) with that of other metals oxides like Zinc-oxide (ZnO-NPs). Also the effect of those nanoparticles on enhancing the activity of different antibiotics belonging to different antimicrobial classes is still under investigation. It is important to note that although there are some reports on the antibacterial activity of Ag-NPs and ZnO-NPs against bacterial pathogens, no available study has been done to evaluate the synergistic potential of those nanoparticles with antibiotics with respect to the known CLSI standards. The present study aims to tested whether the combination of synthesized nanoparticles with the selected antibiotics can pose distinguishing antibacterial efficacy against tested clinical isolates, when compared to antibiotic alone, which might have implications on the control and treatment of infections. The susceptibility of the selected isolates to antibiotic-nano combination according to EUCAST Clinical Breakpoint was performed. Consequently detecting however the isolates became more susceptible to antibiotic after nano-combination or the combination resulted in only slight increase in the inhibition zone whiles the organism still resistant. Furthermore, the nanoparticles were characterized using different chemical techniques including; UV-visible Spectrophotometer, Transmission Electron Microscopy (TEM) and Energy dispersive X-ray analysis (EDX).
Materials and Methods
Isolation and identification of clinical bacterial isolates
A total of twenty five clinical specimens were randomly collected in sterile screw capped containers from patients with burn wound infection and fistulae wounds. The clinical samples used for this study were collected from patients attending different hospitals including; Burn unit of Tanta Emergency Hospital, Mubarak Hospital and El-Salam Hospital in Tanta, Egypt. Each specimen was cultured on blood agar and nutrient agar plates. The growing colonies on these media were subcultured on MacConkey agar medium and Eosin methylene blue agar (EMB-agar). The recovered isolates were subjected to different morphological and biochemical tests for identification to the species level as described by Bergey’s Manual of Determinative Bacteriology 9th edn. [8].
The isolates were identified as Escherichia coli (n=5) and Klebsiella pneumoniae (n=12). Identification of tested isolates was confirmed with an API 20E (bioMérieux) identification kit. Stock cultures were stored in 0.05 M K-Na-phosphate buffer, pH 7.0, containing 15% glycerol at - 20°C.
Antibiotics
For studying the combination effect of metal-nanoparticles with antibiotics; the antibiotics used in our study were selected to cover almost all the different antibiotic classes suitable for Enterobacteriaceae isolates. The antibiotics used with their antimicrobial subclasses along with the discs' potencies are presented in Table 1.
| Antimicrobial class |
Antimicrobial agent |
Disc content |
| Penicillins |
Ampicillin (Am) |
10 µg |
| β-lactam/β-lactamase inhibitor combinations |
Amoxycillin/Clavulanic acid (20/10 µg) (AMC) |
30 µg |
| Cephems |
Cefotaxime (CTX) |
30 µg |
| Monobactams |
Aztreonam (ATM) |
30 µg |
| Carbapenems |
Imipenem (IPM) |
10 µg |
| Aminoglycosides |
Gentamycin (GEN) |
10 µg |
| Quinolones |
Levofloxacin (LEV) |
5 µg |
| Foliate pathway inhibitor |
Trimethoprim-sulfamethoxazole (SXT) |
25 µg |
| Phenicols |
Chloramphenicol (C) |
30 µg |
| Tetracyclines |
Tetracycline (TE) |
30 µg |
Table 1: Antibiotic susceptibility disks (Bioanalyse) along with their codes and potencies.
Chemical synthesis of metal nanoparticles
Synthesis of Ag-NPs by the chemical reduction method: The Ag-NPs used in this study were synthesized using the chemical reduction method [9]. This method involves the reduction of silver nitrate aqueous solution (AgNO3) by using trisodium citrate (C6H5O7Na3) which acts as reducing agent and stabilizing agent [10]. AgNO3 and C6H5O7Na3 (Sigma Aldrich, UK) of analytical grade purity were used as starting materials. In that method, 50 ml of 1 × 10-3 M AgNO3 was heated to boiling. Later, 5 ml of 1% C6H5O7Na3 was added drop by drop to this solution with vigorous mixing. The mixture was heated until color change was evident (pale yellow) indicating the formation of Ag-NPs. The suspension was removed from the heating element and stirred until cooled to room temperature.
The mechanism of reaction could be expressed according to Šileikait? et al. [11] as follows: 2 Ag+ + C6H5O7Na3 + 2 H2O → 4 Ag0 + C6H5O7H3 + 3Na+ + H+ + O2 ↑
Synthesis of ZnO-NPs by precipitation method: ZnO-NPs were synthesized by the precipitation method described by Salahuddin et al. [12] in the Chemistry Department, Faculty of Science, Tanta University. Zinc nitrate hexahydrate (Zn (NO3)2.6 H2O) (0.1 M, solution A) and sodium carbonate (Na2CO3) (0.12 M, solution B) were prepared then solution A was added to solution B drop wise under vigorous stirring. The white precipitate formed was collected by filtration and rinsed with distilled water three times. The solid was then washed with ethanol and dried at 100°C for 6 h.
Characterization of the chemically synthesized nanoparticles
Visual inspection: In case of Ag-NPs, the conversion of the colorless solution of AgNO3 to a pale yellow color after the reduction with sodium citrate clearly indicated the formation of Ag-NPs [9]. While, in case of ZnO-NPs, the formation of a white precipitate from the precipitation of zinc nitrate indicated the formation of ZnO-NPs [12].
UV-Vis spectroscopy: The formation of the nanoparticles and their optical properties were analyzed via UV-visible Spectrophotometer according to the method of Mie [13] at wavelength 300-800 nm with Ag-NPs and 360-500 nm with ZnO-NPs using Shimadzu dual beam spectrophotometer (Model UV 1650 PC) operated at a resolution of 1nm in the Chemistry Department, Faculty of Science, Tanta University, Egypt.
Transmission electron microscopy (TEM): The morphology and the mean size of the synthesized Ag-NPs and ZnO-NPs were measured by JEOL, JEM-100 SX electron microscope (Japan) in Faculty of Medicine, Tanta University. The transmission electron microscopy was operated at 200 kv and the samples were prepared by dropping the nanoparticles onto carbon coated Cu grid underlying tissue paper, leaving behind a film [10].
Energy dispersive x-ray analysis (EDX): In order to detect the elemental composition of the synthesized nanoparticles, their nano-powders were analyzed by EDX [14]. Samples were collected in the powder form and analyzed by EDX (model FEI) in the Chemical War Labs, Armed Forces, Cairo, Egypt.
Assessment of antibiotic susceptibility, resistance pattern and calculating the multiple antibiotics resistance (MAR) index of the tested bacteria
All the isolates were tested for their susceptibility to the selected antibiotics using Disc Diffusion Method [15]. Bacterial inoculum (100 μl) of 1 × 108 CFU/ml for each isolate was used to inoculate Mueller-Hinton agar (MHA). The plates were allowed to set and the selected antibiotic discs were placed using sterile forceps onto the agar plates. The diameters of the inhibition zones were determined and the results were interpreted as susceptible, intermediately resistant or resistant by referring to the Performance Standards for Antimicrobial Susceptibility Testing [16]. The resistance patterns of the isolates were recorded and the MAR index was calculated for each isolate [17,18] as follow:

Determination of the antibacterial efficacy of the synthesized nanoparticles
The antibacterial activity of synthesized Ag-NPs and ZnO-NPs was investigated by well diffusion method using six isolates; (two isolates of E. coli and four isolates of K. pneumoniae) selected randomly as representatives to the different resistant patterns obtained. The tested inoculums were adjusted to concentration 1 × 108 CFU/ml then 100 μl of each inoculum was applied onto the surface of MHA plates free of nanoparticles using sterile cotton swab. The plates were allowed to dry and a sterile well - cutter of diameter 6.0 mm was used to bore two wells in each plate. Subsequently, two volumes (50 μl and 100 μl) of the Ag-NPs or ZnO-NPs suspensions were introduced, respectively into the wells. The plates were allowed to stand for 1 h for the diffusion to take place and then incubated at 37°C for 18 h. After incubation, the diameters of the inhibitory zones (mm) were measured [19]. All the steps were made in triplicates.
Determination of the efficacy of nanoparticles on enhancing the activity of different antibiotics
Only antibiotics to which the selected isolates were found to be resistant were selected for investigation. The antibacterial activities of these antibiotics alone were compared with those with their combinations with Ag-NPs and ZnO-NPs, respectively by disc diffusion method against the selected isolates [5,20]. Bacterial inocula were prepared and the plates were inoculated as mentioned before. The plates were allowed to set and then the standard antibiotics’ discs with and without nanoparticles were placed onto the surface of the inoculated agar plates using sterile forceps. For determining the synergistic effects between antibiotics and nanoparticles, antibiotic discs impregnated with 50 μl of Ag-NPs or ZnO-NPs, respectively were used. Plates were labeled carefully and incubated for 18 h at 37°C. After incubation, the diameters of the inhibitory zones (mm) around the discs were measured. All experiments were conducted in triplicates to confirm the results.
The fold increase in the diameter of inhibition zone of each antibiotic after the combination with Ag-NPs or ZnO-NPs was calculated according to the equation;
The fold increase = (b2-a2)/a2,
Where; (a) is the inhibition zone of antibiotic alone and (b) is the inhibition zone of antibiotic plus nanoparticles [20].
The number of isolates changed from resistant to an antibiotic to intermediately resistant or sensitive to the same antibiotic after the combination with nanoparticles were recorded.
Results and Discussion
Chemical synthesis and characterization of nanoparticles
In this study, two different metals nanoparticles including silver nanoparticles (Ag-NPs) and zinc oxide nanoparticles (ZnO-NPs) were chemically synthesized and analyzed by multiple techniques in order to insure the completion of the nano formation and detection of their characteristics.
Visual inspection
The synthesis of Ag-NPs was detected by visual observation for the conversion of the colorless solution of AgNO3 to a pale yellow color by the reduction with sodium citrate [9]. While ZnO-NPs, was indicated by the formation of a white precipitate (Figure 1) [20].
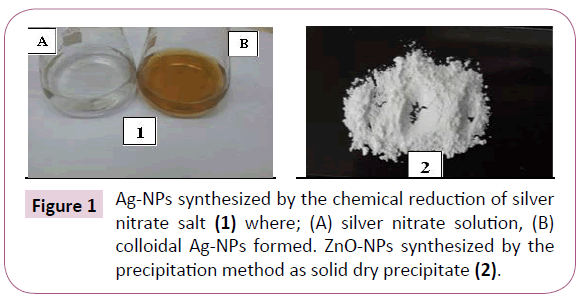
Figure 1: Ag-NPs synthesized by the chemical reduction of silver nitrate salt (1) where; (A) silver nitrate solution, (B) colloidal Ag-NPs formed. ZnO-NPs synthesized by the precipitation method as solid dry precipitate (2).
UV-Vis spectroscopy: The UV–Vis absorption spectrum of the synthesized Ag-NPs is shown in Figure 2 where, a well defined surface plasmon band centered at around 420 nm, characteristic to Ag-NPs was obtained. For ZnO-NPs, Chieng and Loo [21] reported that the broad absorption peak observed in spectrum at 360-380 nm is a characteristic band for the pure ZnO nanoparticles. No other peak was observed in the spectrum which confirms that the synthesized products are ZnO only Figure 3.
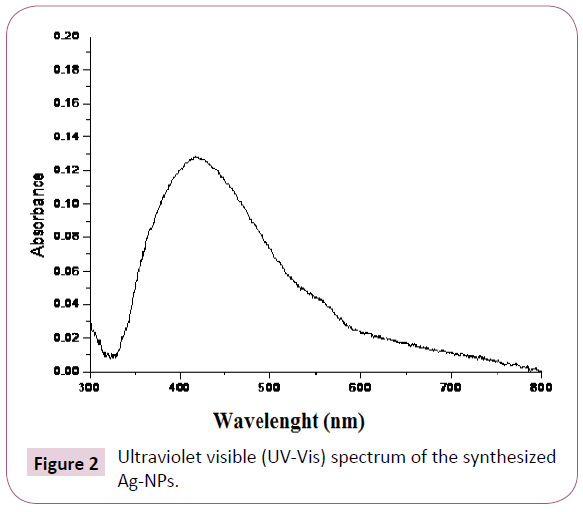
Figure 2: Ultraviolet visible (UV-Vis) spectrum of the synthesized Ag-NPs.

Figure 3: Ultraviolet visible (UV-Vis) spectrums of the synthesized ZnO-NPs.
Energy dispersive x-ray analysis (EDX): The EDX profiles obtained in the present study confirmed the presence of Ag-NPs and ZnONPs with strong signal energy peaks for Ag and Zn atoms in the standard range as shown in Figures 4 and 5.

Figure 4: Energy dispersive X-ray (EDX) analysis of the synthesized Ag-NPs.
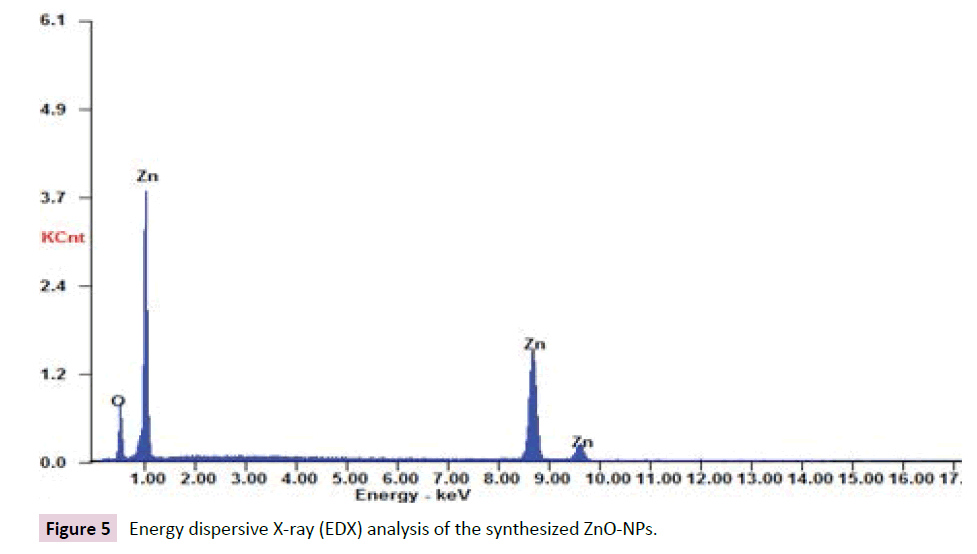
Figure 5: Energy dispersive X-ray (EDX) analysis of the synthesized ZnO-NPs.
Transmission electron microscopy (TEM): The morphology and the average size of the synthesized nanoparticles were analyzed by Transmission Electron Microscopy (TEM). The particles of Ag- NPs synthesized by the chemical reduction method and ZnO-NPs synthesized by precipitation method are well separated single spherical particles without aggregation. The presence of the particles without aggregation clearly insured the efficiency of the techniques used in the synthesizing processes. The average size for the particles was calculated and found to be 12.65 ± 0.55 and 7.6 ± 0.5 nm for Ag-NPs and ZnO-NPs, respectively (Figure 6).
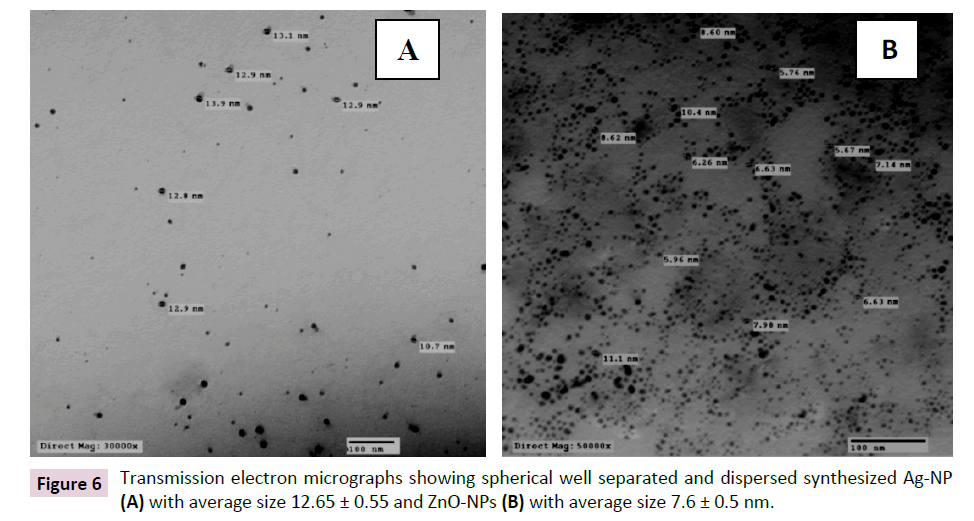
Figure 6: Transmission electron micrographs showing spherical well separated and dispersed synthesized Ag-NP (A) with average size 12.65 ± 0.55 and ZnO-NPs (B) with average size 7.6 ± 0.5 nm.
Antibiotic resistance pattern and MAR index of the isolated bacteria.
In the present study a total of seventeen isolate; E. coli (n=5) and K. pneumoniae (n=12) isolates were recovered from clinical specimens. The susceptibility of the tested isolates was performed against 10 different antibiotics using disc diffusion method. The incidence of resistance to the selected antibiotics was presented in Table 2. It was found that all the isolates were resistant to Ampicillin, Amoxicillin/ Clavulanic acid and Cefotaxime. Moreover, 15 isolates were found to be resistant to Trimethoprim/Sulfamethoxazole followed by Aztreonam (11 isolates) and Chloramphenicol (10 isolates) the lowest resistance among the isolates was obtained against Levofloxacin antibiotic where only 4 isolates were found to be resistant. None of the isolates showed any resistance to Imipenem or Gentamycin. From the obtained data, it was concluded that all the isolates were highly MDR isolates. Abbas et al. [22] investigated the bacterial infections in patients with burn wounds in a burn unit in Hehia General Hospital in Egypt. Total of 160 isolates were recovered; where; S. aureus was the most common pathogen besides P. aeruginosa, K. pneumoniae and S. epidermidis E. coli, Enterobacter cloacae and Citrobacter freundii were also present. Most of the isolates were highly MDR as 100% of the S. aureus isolates were found to be resistant to Ampicillin and Cefazolin while, E. coli and Klebsiella sp. were found to be highly resistant to Tobramycin, Cefoperazone, Amoxicillin-Clavulanic acid, Gentamycin, Cefepime and Amoxicillin (100% of the isolates). Moreover, all the Pseudomonas isolates were found to be resistant to Amoxicillin - Clavulanic acid, Amoxicillin, Tetracycline and Cefepime.
| Antibiotic |
No. of resistant isolates (%) |
| Ampicillin |
17 (100) |
| Amoxicillin/Clavulanic acid |
17 (100) |
| Cefotaxime |
17 (100) |
| Aztreonam |
11 (64.7) |
| Imipenem |
0 (0) |
| Gentamicin |
0 (0) |
| Levofloxacin |
4 (23.5) |
| Trimethoprim/sulfamethoxazole |
15 (88.2) |
| Chloramphenicol |
10 (58.8) |
| Tetracycline |
9 (52.9) |
Table 2: The incidence of resistance of different antibiotics among different isolates.
According to the data in Table 3 it was found that, the tested isolates were found to be heterogeneous in their antibiotic resistance patterns where; the twelve tested isolates had resulted in ten different patterns. The most represented patterns were (I B and II B), each represented by three isolates, while patterns (IA, II A and II E) were represented by two isolates and the other patterns were represented by one isolate. The resistant patterns and the MAR index of the isolates were conducted and the data were recorded in Table 3.
Pattern
Code |
Antimicrobial resistance pattern |
MAR index |
No. of isolates exhibit this pattern |
| I |
A |
Am, AMC, CTX, C, TE |
0.5 |
2 |
| B |
Am, AMC, CTX, ATM, SXT |
3 |
| II |
A |
Am, AMC, CTX, SXT, C, TE |
0.6 |
2 |
| B |
Am, AMC, CTX, ATM, SXT, C |
3 |
| C |
Am, AMC, CTX, LEV, SXT, C |
1 |
| D |
Am, AMC, CTX, ATM, LEV, SXT |
1 |
| E |
Am, AMC, CTX, ATM, SXT, TE |
2 |
| III |
A |
Am, AMC, CTX, LEV, SXT, C, TE |
0.7 |
1 |
| B |
Am, AMC, CTX, ATM, LEV, SXT, TE |
1 |
| C |
Am, AMC, CTX, ATM, SXT, C, TE |
1 |
*Am: Ampicillin; AMC: Amoxicillin/clavulanic acid; CTX: Cefotaxime; ATM: Aztreonam; IPM: Imipenem; GEN: Gentamicin; LEV: Levofloxacin; SXT: Trimethoprim/sulfamethoxazole; C: Chloramphinicol; TE: Tetracycline.
Table 3: Resistance patterns and MAR index of tested isolates.
The antibacterial efficacy of Ag-NPs and ZnONPs against E. coli and K. pneumoniae isolates.
The antibacterial efficacy of both tested nanoaprticles was evaluated against two E. coli and four K. pneumoniae isolates. According to the obtained results, it was found that all the isolates were sensitive to Ag-NPs with zone of inhibition with diameter ranging between 14 and 15 mm except two K. pneumoniae isolates (K2 and K4) that were resistant. On the other hand, the sensitivity of the isolates to ZnO-NPs were found to be less than that to silver one; where only two isolates (E2 and K1) were sensitive to ZnO-NPs while all the other isolates did not show any inhibition zone with ZnO-NPs treatment as shown in Table 4. Generally, there was no difference between the diameter of inhibition zones obtained by 50 and 100 μl of either Ag-NPs or ZnO-NPs Figure 7.
Isolates
Code |
Mean of inhibition zone diameter (mm) ± SD |
| Ag-NPs |
ZnO-NPs |
| 50 μl 100 μl |
50 μl 100 μl |
| E1 |
15 ± 0.12 |
15 ± 0.21 |
0 ± 0.00 |
0 ± 0.00 |
| E2 |
14 ± 0.12 |
14 ± 0.26 |
12 ± 0.17 |
12 ± 0.35 |
| K1 |
15 ± 0.20 |
15 ± 0.25 |
13 ± 0.15 |
13 ± 0.29 |
| K2 |
0 ± 0.00 |
0 ±0.00 |
0 ± 0.00 |
0 ± 0.00 |
| K3 |
15 ± 0.15 |
15 ± 0.20 |
0 ± 0.00 |
0 ± 0.00 |
| K4 |
0 ± 0.00 |
0 ± 0.00 |
0 ± 0.00 |
0 ± 0.00 |
*E: E. coli; K: K. pneumonia; SD: Standard deviation. Each result were in triplicate.
Table 4: Antibacterial activity of Ag-NPs and ZnO-NPs against the selected E. coli and K. pneumoniae isolates.
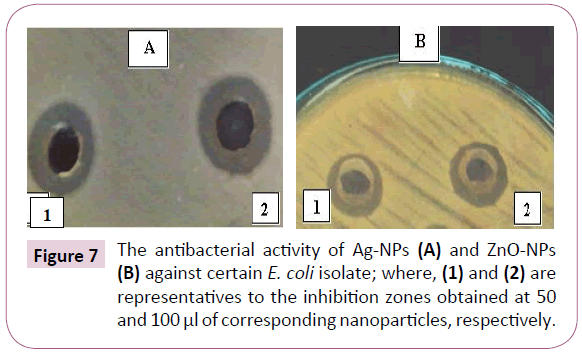
Figure 7: The antibacterial activity of Ag-NPs (A) and ZnO-NPs (B) against certain E. coli isolate; where, (1) and (2) are representatives to the inhibition zones obtained at 50 and 100 μl of corresponding nanoparticles, respectively.
The efficacy of tested nanoparticles on enhancing the activity of different antibiotics
In this experiment, the antibiotics to which the isolates were found to be resistant were selected from the resistant pattern of each isolate and the antibacterial activities of these antibiotics alone were compared with those with their combinations with Ag-NPs or ZnO-NPs using disc diffusion technique. The effect of these nanoparticles was determined as fold increasing in the inhibition zone diameter and the data was represented in Table 5.
| Isolate code |
Tested antibiotic |
Treatment
Type |
Mean of inhibition zone diameter (mm) ± SD |
Fold increase in inhibition zone diameter |
Combination's susceptibility |
| K1 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
15 ± 0.58 |
3.592 |
I |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
0 ± 0.00 |
- |
- |
| AMC + Ag |
15 ± 0.50 |
3.592 |
I |
| AMC + ZnO |
13 ± 0.50 |
2.449 |
R |
| CTX |
CTX only |
9 ± 0.29 |
- |
- |
| CTX + Ag |
13 ± 0.50 |
1.086 |
R |
| CTX+ ZnO |
12 ± 0.00 |
0.778 |
R |
| C |
C only |
0 ± 0.00 |
- |
- |
| C + Ag |
21 ± 1.00 |
8.000 |
S |
| C + ZnO |
0 ± 0.00 |
0.000 |
R |
| TE |
TE only |
0 ± 0.00 |
- |
- |
| TE + Ag |
20 ± 0.58 |
7.163 |
S |
| TE + ZnO |
0 ± 0.00 |
0.000 |
R |
| K2 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
18 ± 0.58 |
5.612 |
S |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
0 ± 0.00 |
- |
- |
| AMC + Ag |
17 ± 1.00 |
4.898 |
I |
| AMC + ZnO |
0 ± 0.00 |
0.000 |
R |
| CTX |
CTX only |
0 ± 0.00 |
- |
- |
| CTX + Ag |
20 ± 0.89 |
7.163 |
R |
| CTX+ ZnO |
0 ± 0.00 |
0.000 |
R |
| ATM |
ATM only |
0 ± 0.00 |
- |
- |
| ATM + Ag |
17 ± 0.50 |
4.898 |
R |
| ATM + ZnO |
0 ± 0.00 |
0.000 |
R |
| SXT |
SXT only |
0 ± 0.00 |
- |
- |
| SXT + Ag |
17 ± 0.00 |
4.898 |
S |
| SXT + ZnO |
0 ± 0.00 |
0.000 |
R |
| E1 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
15 ± 0.50 |
3.592 |
I |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
0 ± 0.00 |
- |
- |
| AMC + Ag |
18 ± 0.58 |
5.612 |
S |
| AMC + ZnO |
11 ± 0.50 |
1.469 |
R |
| CTX |
CTX only |
0 ± 0.00 |
- |
- |
| CTX + Ag |
0 ± 0.00 |
0.000 |
R |
| CTX+ ZnO |
0 ± 0.00 |
0.000 |
R |
| ATM |
ATM only |
0 ± 0.00 |
- |
- |
| ATM + Ag |
15 ± 0.00 |
3.592 |
R |
| ATM + ZnO |
10 ± 0.58 |
1.041 |
R |
| SXT |
SXT only |
0 ± 0.00 |
- |
- |
| SXT + Ag |
17 ± 0.58 |
4.898 |
S |
| SXT + ZnO |
0 ± 0.00 |
0.000 |
R |
| TE |
TE only |
11 ± 0.00 |
- |
- |
| TE + Ag |
22 ± 1.15 |
3.000 |
S |
| TE + ZnO |
11 ± 0.00 |
0.000 |
R |
| K3 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
14 ± 0.00 |
3.000 |
I |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
0 ± 0.00 |
- |
- |
| AMC + Ag |
12 ± 0.76 |
1.939 |
R |
| AMC + ZnO |
0 ± 0.00 |
0.000 |
R |
| CTX |
CTX only |
0 ± 0.00 |
- |
- |
| CTX + Ag |
0 ± 0.00 |
0.000 |
R |
| CTX+ ZnO |
0 ± 0.00 |
0.000 |
R |
| ATM |
ATM only |
9 ± 0.00 |
- |
- |
| ATM + Ag |
11 ± 0.50 |
0.494 |
R |
| ATM + ZnO |
10 ± 0.00 |
0.235 |
R |
| SXT |
SXT only |
0 ± 0.00 |
- |
- |
| SXT + Ag |
15 ± 0.50 |
3.592 |
I |
| SXT + ZnO |
0 ± 0.00 |
0.000 |
R |
| C |
C only |
0 ± 0.00 |
- |
- |
| C + Ag |
14 ± 0.29 |
3.000 |
I |
| C + ZnO |
0 ± 0.00 |
0.000 |
R |
| K4 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
10 ± 0.58 |
1.041 |
R |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
0 ± 0.00 |
- |
- |
| AMC + Ag |
0 ± 0.00 |
0.000 |
R |
| AMC + ZnO |
0 ± 0.00 |
0.000 |
R |
| CTX |
CTX only |
0 ± 0.00 |
- |
- |
| CTX + Ag |
14 ± 0.58 |
3.000 |
R |
| CTX+ ZnO |
0 ± 0.00 |
0.000 |
R |
| LEV |
LEV only |
10 ± 0.00 |
- |
- |
| LEV + Ag |
19 ± 0.50 |
2.610 |
S |
| LEV + ZnO |
11 ± 0.29 |
0.210 |
R |
| SXT |
SXT only |
0 ± 0.00 |
- |
- |
| SXT+ Ag |
14 ± 0.00 |
3.000 |
I |
| SXT+ ZnO |
0 ± 0.00 |
0.000 |
R |
| C |
C only |
0 ± 0.00 |
- |
- |
| C + Ag |
12 ± 0.58 |
1.939 |
R |
| C + ZnO |
0 ± 0.00 |
0.000 |
R |
| TE |
TE only |
0 ± 0.00 |
- |
- |
| TE + Ag |
13 ± 0.29 |
2.449 |
I |
| TE+ Zn |
0 ± 0.00 |
0.000 |
R |
| E2 |
Am |
Am only |
0 ± 0.00 |
- |
- |
| Am + Ag |
14 ± 0.29 |
3.000 |
I |
| Am+ ZnO |
0 ± 0.00 |
0.000 |
R |
| AMC |
AMC only |
10 ± 0.00 |
- |
- |
| AMC + Ag |
19 ± 1.15 |
1.984 |
S |
| AMC + ZnO |
16 ± 1.00 |
1.116 |
I |
| CTX |
CTX only |
0 ± 0.00 |
- |
- |
| CTX + Ag |
13 ± 0.00 |
2.448 |
R |
| CTX+ ZnO |
0 ± 0.00 |
0.000 |
R |
| ATM |
ATM only |
9 ± 0.00 |
- |
- |
| ATM + Ag |
23 ± 0.58 |
5.530 |
S |
| ATM + ZnO |
9 ± 0.00 |
0.000 |
R |
| LEV |
LEV only |
0 ± 0.00 |
- |
- |
| LEV + Ag |
22 ± 0.29 |
8.878 |
S |
| LEV + ZnO |
10 ± 0.29 |
1.041 |
R |
| SXT |
SXT only |
0 ± 0.00 |
- |
- |
| SXT+ Ag |
16 ± 1.00 |
4.224 |
S |
| SXT+ ZnO |
12 ± 0.00 |
1.939 |
I |
| TE |
TE only |
0 ± 0.00 |
- |
- |
| TE + Ag |
12 ± 0.29 |
1.939 |
I |
| TE + ZnO |
0 ± 0.00 |
0.000 |
R |
*E: E. coli; K: K. pneumonia; *Am: Ampicillin; AMC: Amoxicillin/clavulanic acid; CTX: Cefotaxime; ATM: Aztreonam; LEV: levofloxacin; SXT: Trimethoprim/sulfamethoxazole; C: Chloramphenicol; TE: tetracycline; *R: resistant; I: intermediately resistant and S: sensitive; *SD: Standard deviation.
Table 5: Antimicrobial activity of antibiotics alone and in combination with Ag-NPs or ZnO-NPs against selected resistant E. coli and K. pneumoniae isolates.
According to the obtained results, it was clearly observed that Ag-NPs not only have high antibacterial activity but also they show ability to enhance the activity of other antibiotics illustrated as synergistic effect by increasing the inhibition zone diameter around the antibiotic discs supplemented with Ag-NPs. Moreover, the antibacterial activities of the tested antibiotics discs combined with Ag-NPs were higher than that of the same antibiotics with ZnO-NPs as most of antibiotics remained noneffective even after ZnO-NPs combination (Figures 8 and 9).
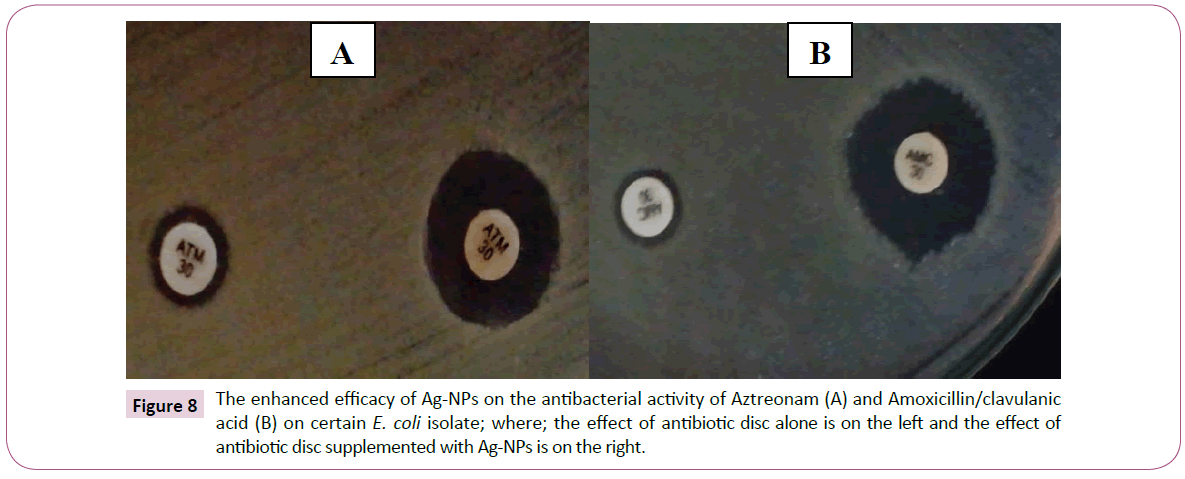
Figure 8: The enhanced efficacy of Ag-NPs on the antibacterial activity of Aztreonam (A) and Amoxicillin/clavulanic acid (B) on certain E. coli isolate; where; the effect of antibiotic disc alone is on the left and the effect of antibiotic disc supplemented with Ag-NPs is on the right.

Figure 9: The enhanced efficacy of ZnO-NPs on the antibacterial activity of Amoxicillin/clavulanic on one of K. pneumoniae isolates where; the effect of antibiotic disc alone on the left and the effect of the antibiotic disc supplemented with ZnO-NPs on the right.
The highest fold increase in the inhibition zone by Ag-NPs combination (8.878) was observed with Levofloxacin against isolate E2 followed by that of Chloramphenicol (8.000) against isolate K1. In addition, the highest fold increase with ZnO-NPs combination was (1.939) with Amoxicillin/Clavulanic acid and Trimethoprim/Sulfamethoxazole against isolate E2.
The obtained inhibition zone diameters were interpreted to sensitive, intermediately resistant or resistant according to EUCAST Clinical Breakpoint [16]. The change in the susceptibility of each isolate to any of the tested antibiotics was determined in the presence of either Ag-NPs or ZnO-NPs as shown in Table 5.
For the ease of comparison, Table 6 was constructed to summarize the efficacy of tested combinations against the tested isolates.
| Antibiotic |
No. of tested isolates |
Antibiotic-Nano combination |
No. (%) of isolates changed to intermediately sensitive |
No. (%) of isolates changed to sensitive |
| Am |
6 |
Amp – Ag-NPs |
4 (66.7) |
1 (16.6) |
| Amp – ZnO-NPs |
0 (0) |
0 (0) |
| AMC |
6 |
AMC – Ag-NPs |
2 (33.3) |
2 (33.3) |
| AMC – ZnO-NPs |
0 (0) |
0 (0) |
| CTX |
6 |
CTX – Ag-NPs |
0 (0) |
0 (0) |
| CTX – ZnO-NPs |
0 (0) |
0 (0) |
| C |
3 |
C – Ag-NPs |
1 (33.3) |
1 (33.3) |
| C – ZnO-NPs |
0 (0) |
0 (0) |
| TE |
4 |
TE – Ag-NPs |
2 (50) |
2 (50) |
| TE – ZnO-NPs |
0 (0) |
0 (0) |
| ATM |
4 |
ATM – Ag-NPs |
0 (0) |
1 (25) |
| ATM – ZnO-NPs |
0 (0) |
0 (0) |
| SXT |
5 |
SXT – Ag-NPs |
2 (40) |
3 (60) |
| SXT – ZnO-NPs |
0 (0) |
0 (0) |
| LEV |
2 |
LEV – Ag-NPs |
0 (0) |
2 (100) |
| LEV – ZnO-NPs |
0 (0) |
0 (0) |
*Am: Ampicillin; AMC: Amoxicillin/clavulanic acid; CTX: Cefotaxime; C: Chloramphenicol; TE: Tetracycline; ATM: Aztreonam; SXT: Trimethoprim/sulfamethoxazole; LEV: levofloxacin.
Table 6: Summary of the efficacy of different antibiotics-nanoparticles combinations on the susceptibility of tested isolates.
According to the obtained it was clearly demonstrated that Ag- NPs are able to interestingly enhance the efficiency of some antibiotics. Among six isolates tested with Ampicillin, four isolates had been changed to intermediately resistant, one isolate had been changed to sensitive while only one isolate still resistant. In addition, for Amoxicillin/Clavulanic acid; two isolates had been changed to sensitive, two isolates two intermediately resistant and two isolates still resistant. Moreover, the Tetracycline and Trimethoprim/Sulfamethoxazole combination with Ag-NPs had resulted in increasing the susceptibility of all tested isolates to either sensitive or intermediately resistant. However, the combination between Ag-NPs and other antibiotics such as Cefotaxime resulted in increasing in inhibition zone diameter but it still in the resistant range indicating that the effect of Ag-NPs could be varied according to antibiotic class and mode of action.
These finding agreed with that obtained by Deng et al. [23] who studied the mechanism of synergistic activity between Ag-NPs and antibiotics (Ampicillin, Penicillin, Enoxacin, Kanamycin and Tetracycline) on MDR Salmonella typhimurium DT 104. They reported that Enoxacin, kanamycin, Neomycin and Tetracycline showed synergistic growth inhibition and antibiotic - nano complexes formation was also detected. While, Ampicillin and Penicillin neither form complexes nor showed synergism with Ag-NPs. They explained that the suggested mechanism of this synergism with some antibiotics is that firstly, antibiotic−AgNPs complex are formed then they interacts more strongly with the bacterial cells and causes more Ag+ release, thus creating a temporal high concentration of Ag+ near the bacteria cell wall that leads to growth inhibition of the bacteria.
Our findings about the enhancing activity of silver agreed with that of Sindhu et al. [20] who compared the activity of Gentamycin and Chloramphenicol antibiotics alone with that with Ag-NPs. They reported that the antibacterial activity of both antibiotics increased in the presence of Ag-NPs against the tested strains. Also, Kora and Rastogi [24] studied the effect of chemically synthesized Ag-NPs capped with three different capping agents; citrate, sodium dodecyl sulfate (SDS) and poly vinyl pyrrolidone (PVP) on the antibacterial activity of Streptomycin, Ampicillin, and Tetracycline against E. coli and S. aureus. They reported that the highest percentage of enhancement in activity against E. coli was obtained in combination of Ampicillin with PVP and SDS capped nanoparticles; while, Streptomycin combination with PVP capped nanoparticles showed the highest activity against S. aureus.
Furthermore, the observed lower activities of ZnO-NPs in our study agree with the findings of Meruvu et al. [25] who investigated the antibacterial activity of ZnO-NPs combinations with Gentamycin, Nitrofurantoin and Ciprofloxacin against E. coli where, the results showed that the activity of nanoparticles alone was lower than that of antibiotics alone than that of antibiotics- ZnO-NPs combination.
Conclusion
The increasing antimicrobial resistance among bacterial strains is a great threat that needs rapid and serious actions to be taken. Due to their unique antibacterial activity; silver nanoparticles could be evaluated as alternatives to the traditional antibiotics or used to enhance the efficacy of certain antibiotics.
Acknowledgment
Authors thank Dr. Shereen F. Gheida, Department of Dermatology and Venereology, Faculty of Medicine, Tanta University, Egypt, Who have helped in collecting samples from patient.
20624
References
- Health Policy Brief (2015) Antibiotic Resistance. Health Affairs 12: 1-7.
- Melakea NA, Eissaa NA, Keshkb TF, Sleema AS (2015) Prevalence of multidrug-resistant bacteria isolated from patients with burn infection. Menoufia Med J 28: 677-684.
- Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24: 71-109.
- Naqvi SZH, Kiran U, Ali MI, Jamal A, Hameed A, et al. (2013) Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug- resistant bacteria. Int J Nanomedicine 8: 3187-3195.
- Singh R, Nawale LU, Arkile M, Shedbalkar UU, Wadhwani SA, et al. (2015) Chemical and biological metal nanoparticles as antimycobacterial agents: A comparative study. I J Anti Mic Ag 46: 183-188.
- Rashid BZ, Omar RA, Salih SJ (2016) Characterization and antimicrobial efficiency of silver nanoparticles based reduction method. Int J Curr Microbiol App Sci 5: 802-810.
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Genus Acetobacter and Gluconobacter. Bergey’s manual of determinative bacteriology. 9th edn. Williams and Wilkens, MD USA 14: 668-675.
- Fang J, Zhang C, Mu R (2005) The study of deposited silver particulate films by simple method for efficient SERS. Chem Phys Lett401: 271-275.
- Zawrah MF, Abd El-Moez SI (2011) Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life sci J 8: 37-44.
- Šileikait? A, Prosy?evas I, Puišo J, Juraitis A, Guobien? A (2006) Analysis of silver nanoparticles produced by chemical reduction of silver salt Solution. Mater Sci Medzg 12: 287-291.
- Salahuddin NA, El-Kemary M, Ibrahim EM (2015) Synthesis and characterization of ZnO nanoparticles via precipitation method: Effect of annealing temperature on particle size. J Nanosci Nanotechnol 5: 82-88.
- Mie G (1908) Contributions to the optics of turbid media, especially colloidal metal solutions. Ann. Phys 25: 377-445.
- Vijayakumara M, Priyab K, Nancy FT, Noorlidaha A, Ahmed ABA (2013) Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crop Pro 41: 235- 240.
- Bauer AW, Kirby WMM, Sherris JC, Truck M (1966) Antibiotic susceptibility testing by a standardized disk method. Amer J Clin Path 45: 493-496.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) (2012) Antimicrobial activity 22.
- Olayinka A, Olayinka B, Onile B (2009) Antibiotic susceptibility and plasmid pattern of Pseudomonas aeruginosa from the surgical unit of a University teaching hospital in north central Nigeria. Int J Med Sci 1: 79-83.
- Jayaraman S, Manoharan M, Illanchezian S, Sekher R, Sathyamurthy S (2010) Plasmid analysis and prevalence of multidrug resistant Staphylococcus aureus reservoirs in Chennai city, India. Asian J Pharmacol Life Sci 10: 117-125.
- Lee SM, Song KC, Lee BS (2010) Antibacterial activity of silver nanoparticles prepared by a chemical reduction method. Korean J Chem Eng 27: 688-692.
- Sindhu PD, Mukherjee A, Chandrasekaran N (2013) Synergistic effect of biogenic silver nanocolloid in combination with antibiotics: A potent therapeutic agent. Int J Pharm Pharm Sci 5: 292-295.
- Chieng BW, Loo YY (2012) Synthesis of ZnO nanoparticles by modified polyol method. Mater Lett 73: 78-82.
- Abbas HA, El-Masry EM, Shaker GH, Mohsen I (2013) Bacterial etiology and antimicrobial resistance of burn wound infections in a burn unit in Hehia general hospital in Egypt. Int J Biol Pharm Res 4: 1251-1255.
- Deng H, McShan D, Zhang Y, Sinha SS, Arslan Z, et al. (2016) Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol 50: 8840-8848.
- Kora AJ, Rastogi L (2013) Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model Gram-negative and Gram-positive bacteria. Bioinorg Chem Appl 8: 319-355.
- Meruvu H, Vangalapati M, Chippada SC, Rao Bammidi SR (2011) Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. Rasayan J Chem 4: 217-222.















