Background: Reactive oxygen species (ROS) have deleterious impact on sperm functional parameters and DNA damage in spermatozoa, both invivo and in-vitro. During sperm preparation for assisted reproductive technologies (ART) additional ROS produces, which lowers the rate of live birth, especially when sperm with DNA damage is used in ART.
Aim: The present study aimed to correlate the concentration of exogenous H2O2 with sperm motility and to see the effect of H2O2 on seminal plasma LPO, catalase and SOD level.
Method and Material: 146 subjects were primarily recruited for the present investigation from the Division of Infertility, Department of Urology, SMS Hospital, Jaipur, Rajasthan, India. Based on sperm motility the semen samples were classified in two groups, (i) semen samples with more than 50% motility graded to normal, and (ii) sperm motility less than 50% graded to asthenozoospermia group.
Results: There was no significant difference in sperm motility and seminal plasma LPO, catalase and SOD levels at 300 μM H2O2 concentration. Sperm motility was significantly (p<0.05) decline within 10 min. of incubation at 600 μM H2O2 concentration. The levels of LPO, catalase and SOD was significantly (p<0.001) increased in 30 min. at 600 μM H2O2 in astenozoospermic group as compared to normal group which was highly significant at 1200 μM H2O2. Exogenous H2O2 was detrimental to motility and resulted in a significant increase in overall ROS.
Conclusion: As demonstrated in the present study that H2O2, one of the most toxic oxygen species, has the ability to alter sperm functions significantly at 600 μM concentration of H2O2. The study confirmed a need to reduce exogenous ROS production during ART. The in-vitro protocol carried out in the present study is simple to execute and appears to be a novel approach.
Keywords
Hydrogen peroxide; Motility; Oxidative stress; LPO; Catalse; SOD
Introduction
Reactive oxygen species (ROS) molecules in general are highly disruptive to cellular function, and one of the factor in male infertility. Oxidative stress (OS) arises as a consequence of excessive ROS production and/or impaired antioxidant defense mechanisms [1]. Owing to their deleterious effects on human spermatozoa, excessive ROS must be continuously inactivated to keep only a small amount necessary to maintain normal cell function [2,3]. Bize et al. and Griveau et al. [4,5] suggested that H2O2 play a significant role during the process of capacitation, possibly by inducing membrane reorganization to facilitate the fusion that takes place during exocytosis of the acrosomal contents. When cells are exposed to external H2O2, inside the cells provide the driving force for setting up a gradient across the plasma membrane [6]. Exogenous addition of H2O2 has been shown to increase intracellular ROS levels in spermatozoa [7]. Oxidative stress is not routinely checked in andrology lab, because of its cost and complexity of testing and the lack of a single standardized measure of oxidative stress. The assessment of quality of sperm DNA has become essential with the increase in the use of assisted reproductive techniques (ART) in infertility managment. In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) techniques bypass several natural selection barriers that are present throughout the male and female reproductive tracts until the sperm enters the oocyte [8]. Investigations have shown disorder results into failure of ICSI and intrauterine insemination (IUI) and one of the main causes of defective offsprings [9]. The rate of live birth is low especially when sperm with DNA damage is used in ART.
This experiment focused on H2O2, which are important intracellular signaling molecules that are required at physiological levels to aid in sperm capacitation, motility and fertilization [10,11]. First it was aimed to see the effects of the in-vitro addition of increasing concentrations of exogenous H2O2 on sperm motility and Lipid Peroxidation (LPO), Catalase and Superoxide Dismutase (SOD) level in seminal plasma. Secondly, to develop a simple and quick method for the assessment of sperm susptibility to oxidative damage.
Methods and Materials
Subject recruitment
Five hundred infertile couples were selected for initial screening. After exclusion of infertility through female factors, males above 45 years, aspermic patients, any known reproductive pathology (e.g. genital tract infections, prostatitis, epididymitis, etc.) or any hormonal therapy in the last six months and patients with erectile dysfunction, 146 subjects were primarily recruited for the present investigation from the Division of Infertility, Department of Urology, SMS Hospital, Jaipur, Rajasthan, India. All the subjects signed an informed consent form in order to undergo a full medical consultation, clinical examination, sample collection and relevant biochemical testing. Clinical examination of all the subjects was carried out and information on age, health problems, history of infertility in the family, height, weight, etc. was recorded. The study was approved by the institute ethical committee (IEC).
Collection of semen samples
Semen samples were collected by masturbation into a clean sterile sample collection vial, under aseptic conditions. Subjects were instructed to abstain for at least 48 hours prior to collection the semen sample. The samples were liquefied for at least 20 minutes in a water bath at 37°C, but no longer than 1 hour prior to performing a routine semen analysis.
Semen characteristics
Semen analysis was performed within one hour after collection of semen. Semen liquefaction, colour, pH, appearance, consistency, agglutination, semen volume and sperm motility was carried out [12].
Tests to assess sperm susceptibility to oxidative stress
A total of 152 semen samples from infertile subjects possessing sperm concentration more than 8 million/mL were used for present study. Based on sperm motility the semen samples were classified in two groups, (i) semen samples with more than 50% motility graded to normal and (ii) sperm motility less than 50% graded to asthenozoospermia group [12].
A simple protocol was followed to assess sperm susceptibility to oxidative stress on sperm motility. The different concentrations, viz., 300, 600 and 1200 μM of H2O2 were prepared in phosphate buffer (pH 7.0) and one mL of seminal plasma was added. Semen sample without H2O2 served as control in the study. (LPO), catalse and superoxide dismutase (SOD) were examined following 10, 20, 30 min. of incubation periods at 37 °C.
Assay for lipid peroxidation (LPO)
In 0.2 mL of incubated seminal plasma, 1.5 mL of acetic acid, 0.2 mL of 8% sodium dodecyl sulphate (SDS) and 0.1 mL of butylated hydroxy toluene (BHT) was added and after mixing of above 1.5 mL of 0.8% thiobarbituric acid (TBA) solution was added. The reaction mixture was incubated in a boiling water bath for one hour. After cooling at room temperature 3 mL of n-butanol was added and centrifuged at 10,000 rpm for 15 min. A clear supernatant obtained after centrifugation was used for measurement of the absorbance at 532 nm against reagent blank [13].
Catalase
In a cuvette containing 2.8 mL of phosphate buffer (pH 7.6) and 0.5 mL of H2O2, 100 μL incubated seminal plasma was added and mixed thoroughly. Initial optical density was adjusted at 0.5 and 0.6 absorbance units at the wavelength of 240 nm. The decrease in absorbance was recorded after every minute for a total period of 3 min [14].
Superoxide dismutase (SOD)
A 100 μL incubated seminal plasma was add in a cocktail mixture of phosphate buffer (pH 7.4), hydroxylamine hydrochloride (HAC) (100 mM), α-methionine (20 nM), EDTA (50 μM), Triton x-100 (1%) and riboflavin (100 μM). The mixture was incubated for a period of 10 min. in a SOD box with a fluorescent light. After incubation 1 mL of Greiss reagent (0.1% napthylethylene diamine + 1% sulphanilamide in 5% orthophosphoric acid) was added and optical density (OD) was measured against blank at 543 nM [15].
Statistical analysis
Values are represented as mean ± standard deviation (SD). Oneway analysis of variance (ANOVA) was employed for statistical comparison. The difference between means was analyzed by Holm-Sidak multiple comparison test to detect the inter-group difference by using the statistical software SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). The P value less than 0.05 were considered as significant. Relationship between two variables was determined by Karl Pearson’s coefficient of correlation.
Results
Semen analysis revels no significant difference in physical parameters i.e., semen liquefaction, pH, color, order and semen volume in both the groups. Sperm motility was significantly (p<0.05) decreased at 600 μM H2O2 concentration in 10 min. of incubation period. Following 60 min. of incubation period all sperms were found to be immotile at all H2O2 concentrations in both poor and normal groups as compared to control (Figure 1). Various indicators of oxidative stress in seminal plasma were evaluated from normal and asthenozoospermia category. An increment in the seminal plasma LPO was noticed at all incubation periods at 300, 600 and 1200 μM H2O2 concentrations in both the asthenozoopsermic and normal groups. However, the levels of LPO found to be significantly increased at (600 μM: p<0.05; and 1200 μM: p<0.001) following 30 min. of incubation period in astenozoospermic group as compared to normal group with their respective control (Figure 2 a-b). In astenozoospermic group the levels of catalase and SOD in seminal plasma was found to be significantly enhanced at 300 (p<0.05) and at 600 μM and 1200 μM of H2O2 concentration, which was drastically increased (p<0.001) following 30 min. of incubation period with respective control (Figure 3 and 4 a-b).
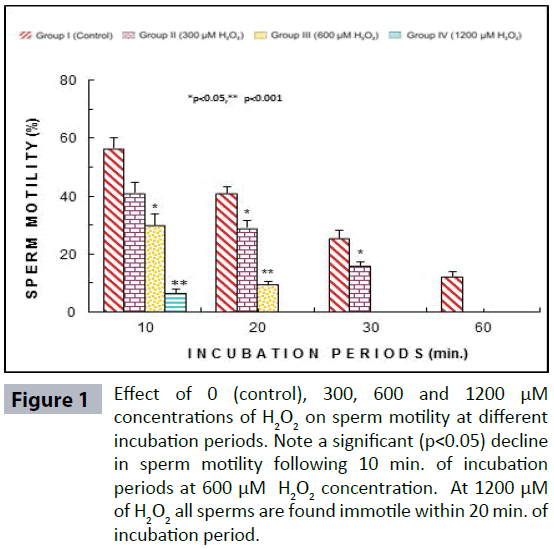
Figure 1: Effect of 0 (control), 300, 600 and 1200 μM concentrations of H2O2 on sperm motility at different incubation periods. Note a significant (p<0.05) decline in sperm motility following 10 min. of incubation periods at 600 μM H2O2 concentration. At 1200 μM of H2O2 all sperms are found immotile within 20 min. of incubation period.
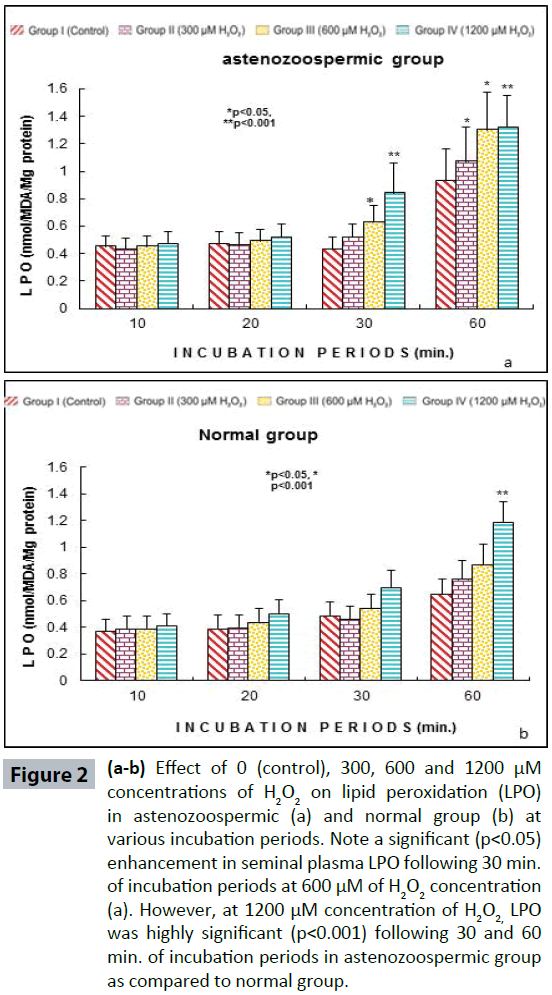
Figure 2(a-b): Effect of 0 (control), 300, 600 and 1200 μM concentrations of H2O2 on lipid peroxidation (LPO) in astenozoospermic (a) and normal group (b) at various incubation periods. Note a significant (p<0.05) enhancement in seminal plasma LPO following 30 min. of incubation periods at 600 μM of H2O2 concentration (a). However, at 1200 μM concentration of H2O2, LPO was highly significant (p<0.001) following 30 and 60 min. of incubation periods in astenozoospermic group as compared to normal group.
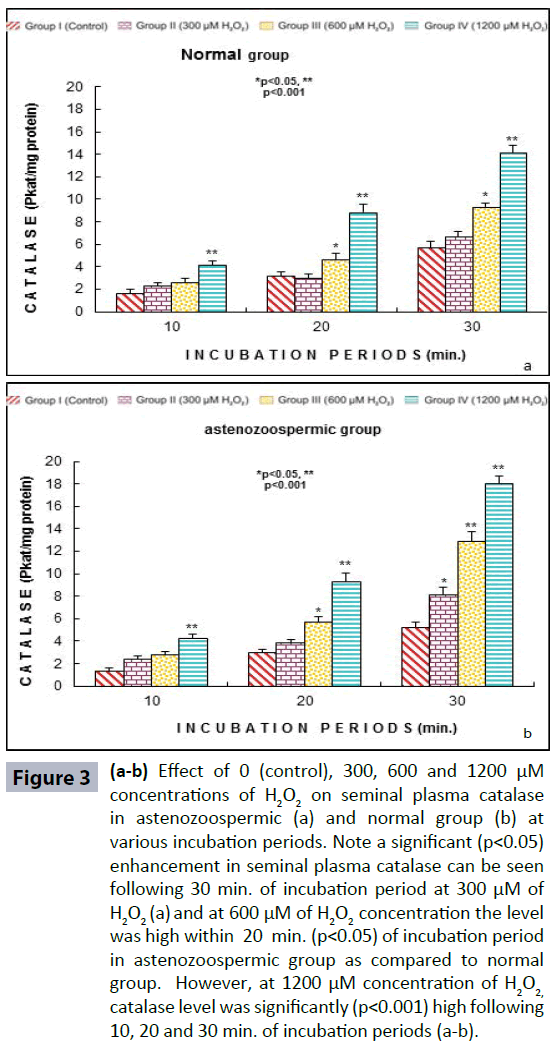
Figure 3 (a-b): Effect of 0 (control), 300, 600 and 1200 μM concentrations of H2O2 on seminal plasma catalase in astenozoospermic (a) and normal group (b) at various incubation periods. Note a significant (p<0.05) enhancement in seminal plasma catalase can be seen following 30 min. of incubation period at 300 μM of H2O2 (a) and at 600 μM of H2O2 concentration the level was high within 20 min. (p<0.05) of incubation period in astenozoospermic group as compared to normal group. However, at 1200 μM concentration of H2O2, catalase level was significantly (p<0.001) high following 10, 20 and 30 min. of incubation periods (a-b).
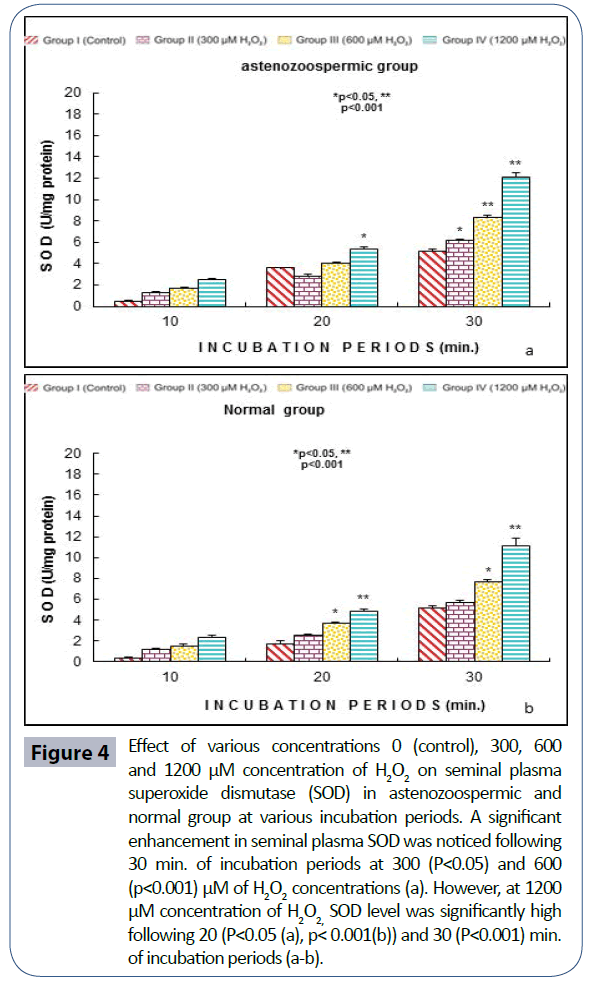
Figure 4: Effect of various concentrations 0 (control), 300, 600 and 1200 μM concentration of H2O2 on seminal plasma superoxide dismutase (SOD) in astenozoospermic and normal group at various incubation periods. A significant enhancement in seminal plasma SOD was noticed following 30 min. of incubation periods at 300 (P<0.05) and 600 (p<0.001) μM of H2O2 concentrations (a). However, at 1200 μM concentration of H2O2, SOD level was significantly high following 20 (P<0.05 (a), p< 0.001(b)) and 30 (P<0.001) min. of incubation periods (a-b).
Discussion
The risk of oxidative stress affecting sperm function may come from factor present either in the semen or in the secretion of female genital tract the relevance of exposing the sperms to an additional oxidative stress comes from the fact that sperms might have to withstand similar stress conditions on the females reproductive tract [16]. During their ascent through the female reproductive tract, spermatozoa are separated from seminal plasma and can be more susceptible to oxidative damage. Spermatozoa can retain their physiological function within the female tract for several days after copulation [17] while they interact with different female fluids, such as the oviductal fluid.
The female fluids can contribute to the redox status of the sperm environment. Exposure to semen elicits an inflammatory response in the female reproductive tract resulting in leukocyte recruitment and cytokine release [18-20]. In addition, it could be though that any inflammatory process of the female reproductive tract, which induces the production of highly toxic ROS, could affect sperm DNA integrity [21].
High levels of OS levels are not only cause for male factor infertility but prolong to play an critical role in low birth rates, because of DNA induced damage in spermatozoa caused by the induced OS. The result obtained supports our assumption that exogenous H2O2 should adversely affect sperm motility parameters and corresponds to the results of other studies [22,23]. An excessive accumulation of ROS such as superoxide radicals (O2 -), or hydrogen peroxide (H2O2) can cause sperm plasma membrane, mitochondrial and nuclear DNA damage in human [24], stallion [25] and bull [26]. Excessive ROS levels have been linked to lipid peroxidation of the sperm plasma membrane fluidity, structure and function [27,28]. The lipid peroxidation reaction results in changes in sperm membrane fluidity, loss of membrane integrity as well as irreversible loss of sperm motility [29]. Lipid peroxidation has been reported to reduce the ability of human spermatozoa to penetrate zona free hamster oocytes [30] and is also related to DNA strand breaks [31]. Seminal plasma from astenozoospermic category showed a significant increase in the activities of catalase and SOD. These results are inconformity with the earlier observations [23,32]. The catalase activity of seminal plasma was significantly greater in those samples that produced ROS. This extra cellular catalase, however, would not have much impact on the sperm antioxidant status. The LPO measurements are relevant because major loss of sperm function may occur with minimal damage to the membranes that envelope the sperm and intracellular sperm compartments. The adverse effects of exogenous H2O2 on sperm motility exemplify the importance of minimizing of ROS introduction during sperm preparation for various ART procedures, especially when spermatozoa are removed from the seminal plasma during washing procedure. Because sperm preparation is necessary to ART procedures, maintaining sperm integrity is vital to the success of the procedure.
Conclusion
The in-vitro protocol carried out in the present study is simple to execute and appears to be a novel approach. The incorporation of such a test into the routine andrology laboratory will be an important step for the future of male infertility practice. Consequently, more research is needed to continue to develop methods to accurately diagnose and prevent DNA damage of spermatozoa used in ART and minimize the production of exogenous OS during sperm preprations. As demonstrated in the present study that H2O2, one of the most toxic oxygen species, has the ability to alter sperm functions significantly at 600 μM concentration of H2O2. These parameters could help in distinction and treatment of male infertility especially in idiopathic cases.
8308
References
- Makker K, Agarwal A, Sharma R (2009) Oxidative stress & male infertility.Indian J Med Res 129: 357-367.
- Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility.Urology 48: 835-850.
- Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction.FertilSteril 79: 829-843.
- Bize I, Santander G, Cabello P, Driscoll D, Sharpe C (1991) Hydrogen peroxide is involved in hamster sperm capacitation in vitro.BiolReprod 44: 398-403.
- Griveau JF, Renard P, Le Lannou D (1994) An in vitro promoting role for hydrogen peroxide in human sperm capacitation.Int J Androl 17: 300-307.
- Antunes F, Cadenas E (2000) Estimation of H2O2 gradients across biomembranes.FEBS Lett 475: 121-126.
- Mahfouz R, Aziz N, Sharma R, Bykova M, Sabanegh E, et al. (2008) Assessment of intracellular human sperm reactive oxyzen species after hydrogen peroxide exposure using four probes. FertilSteril 90: 320-321.
- Agarwal A, Allamaneni SS (2004) Role of free radicals in female reproductive diseases and assisted reproduction.Reprod Biomed Online 9: 338-347.
- deLamirande E, Gagnon C (1993) Human sperm hyperactivation in whole semen and its association with low superoxide scavenging capacity in seminal plasma.FertilSteril 59: 1291-1295.
- Dada R, Gupta NP, Kucheria K (2004) Yqmicrodeletions--azoospermia factor candidate genes and spermatogenic arrest.J Biomol Tech 15: 176-183.
- Zini A, De Lamirande E, Gagnon C (1995) Low levels of nitric oxide promote human sperm capacitation in vitro.J Androl 16: 424-431.
- WHO (1999) Laboratory Manual for the Examination of the Human Semen and Sperm Cervical Mucus Interaction. Cambridge University Press, New York, United States.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal’s tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Aebi H (1974) Catalase. In: Methods in Enzymology, Bregmeyer HU (Ed.) Acadimic Press, New York, pp 673-678.
- Das K, Samanta L,Chainy GBN (2000) A modified spectrophotometric assay for superoxide dismutase using nitrite formation by superoxide radicals. IJBB 37: 201-204.
- Chaki SP,Misro MM, ChoudhuryL, Upreti K, Gautam D, et al. (2003) Use of hydrogen peroxide to assess the sperm suseptiblity to oxidative stress in subjects presenting a normal semen profile. Int. J. Androl 27: 82-87.
- Suarez SS, Pacey AA (2006) Sperm transport in the female reproductive tract.Hum Reprod Update 12: 23-37.
- Troedsson MH, Desvousges A, Alghamdi AS, Dahms B, Dow CA, et al. (2005) Components in seminal plasma regulating sperm transport and elimination.AnimReprodSci 89: 171-186.
- Robertson SA (2007) Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs.J AnimSci 85: E36-44.
- Lea RG, Sandra O (2007) Immunoendocrine aspects of endometrial function and implantation.Reproduction 134: 389-404.
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA (2000) Reactive oxygen species, cell signaling, and cell injury.Free RadicBiol Med 28: 1456-1462.
- Ramos L,WetzelsAM (2001) Low rates of DNA fragmentation in selected motile human spermatozoa assessed by the TUNEL assay. Hum Reprod 16: 1703-1707.
- Misro MM, Choudhury L, Upreti K, Gautam D, Chaki SP, et al. (2004) Use of hydrogen peroxide to assess the sperm susceptibility to oxidative stress in subjects presenting a normal semen profile.Int J Androl 27: 82-87.
- Aitken RJ, Krausz C (2001) Oxidative stress, DNA damage and the Y chromosome.Reproduction 122: 497-506.
- Baumber J, Ball BA, Linfor JJ, Meyers SA (2003) Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa.J Androl 24: 621-628.
- Chatterjee S, Gagnon C (2001) Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing.MolReprodDev 59: 451-458.
- Allamaneni SS, Agarwal A, Nallella KP, Sharma RK, Thomas AJ, et al. (2005)Chaercterization of oxidative stress status by evaluation of reactive oxygen species levels in whole semen and isolated spermatozoa. FertilSteril 83: 800-803.
- Mahfouz R,du Plessis SS, Aziz N, Sharma R, Sabanegh E(2010). Sperm viability, Apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. FertilSteril 93:814-821
- Storey BT (1997) Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa.Mol Hum Reprod 3: 203-213.
- Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FCW (1989) Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl 10: 214-220.
- Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC (2000) The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 21: 895-902.
- Zini A, de Lamirande E, Gagnon C (1993) Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa.Int J Androl 16: 183-188.









