Key words
|
| |
| Butea Monosperma, Hexane: Ethanol extract, Multi drug resistant bacteria, Phytochemicals |
| |
INTRODUCTION
|
| |
| Since ancient times, plants have been model source of medicines as they are a reservoir of chemical agents with therapeutic properties. The general population is increasingly using herbal medicines as dietary supplements to relieve and treat many different human disorders. Herbs and spices are an important part of the human diet. They have been used for thousands of year to enhance the flavour, colour and aroma of food. In addition to boosting flavour, herbs and spices are also known for their preservative and medicinal value, which forms one of the oldest sciences. Yet it is only in recent years that modern science has started paying attention to the properties of spices1. |
| |
| Resistance to antibiotics is becoming a difficult problem in the management of infections caused by Gram-positive bacteria2. The situation is particularly critical for treatment of infections caused by Staphylococcus aureus where methicillin-resistant (MRSA) and vancomycin-intermediate resistant (VISA) strains have emerged, which are also frequently resistant to multiple classes of antibiotics3. The recent report of an MRSA isolate resistant to the new oxazolidinone antimicrobial linezolid4. It is a further disturbing trend in the evolution of antimicrobial resistance in staphylococci. There is therefore a need to discover and develop new approaches for combating S. aureus5. |
| |
| Butea monosperma (Lam) is a deciduous tree, belongs to family fabaceae, which grows up to 15 m in height and 1.5- 1.8 m in girth, with a crooked trunk. Bark light- brown or bluish grey, yielding a ruby-red vitreous gum. Wood white or yellowish–brown, often becoming grey or grayish- brown. Leaves 3- foliolate, large, unequal, 10.2-20.4 cm. Flowers borne in racemes, brilliant orange red, 3.8-5.1 cm long. Lower calyx-teeth deltoid. Pods silvery- white, broad dehiscing by one suture. Seeds flat, elliptic, reddishgrey, 3.2cm6. |
| |
| Butea monosperma Lam is a wild crop and grows in most parts of India as a tree. It is reputed in systems of medicines as the various parts of the plant Butea monosperma has been used traditionally for many of the diseases like anti-inflammatory, antimicrobial, anthelmintic, antidiabetic, diuretic, analgesic, antitumor, anticancer, astringent etc7. The leaves and seeds are useful as, in hemorrhage, astringent, diuretic and have anti-implantation and antiovulatory properties8. Flowers have aphrodisiac and tonic properties. Bark are used in tumors, bleeding piles, ulcers and have inhibitory action against E.coli and Micrococcus pyrogens. Roots are used to cure night blindness. Chemical component of Butea monosperma are alkaloids and recently reported Euphane triterpenoid ester and pterocarpan. Seed contains palasonin,-d-methyl cantharidin, α-amyrin, β-sitosterol and alkaloid- monospermine. Glycerides of palmitic, stearic, linoceric, oleic and linoleic acids, proteolytic and lipolytic enzymes. While bark contains tannins and gum (Butea gum), leucocyanidin and its tetramer procyanidin, gallic acid and mucilaginous material. Its flowers contain isobutin, coreoopsin, monospermoside and their isoderivatives sulphurein, palastrin9. |
| |
MATERIALS AND METHODS
|
| |
|
Plant Material
|
| |
| Leaves of Butea monosperma plant was collected from local region of Narsapur, District of Medak, Andhra Pradesh, India in the month of Jun 2010. The botanical identity was con-firmed by a botanist Prof T.Mohana Department of Botany, Government Mehbubia Junior College, Gunfoundry, Hyderabad. (Reference No: 3/2010), Gentamicin which used as a standard in this experiment was purchased from local market (manufactured by Concord Drugs Limited, batch number 91215) All reagents and chemicals used were AR grade. |
| |
|
Preparation of Extracts
|
| |
| 5 Kg of leaves of Butea monosperma was crushed to coarse powder and passed through sieve # 44. The sieved powder was stored in air tight, high density poly ethylene containers before extraction. Extraction was performed by using soxhlet apparatus (12 hours), carried out first with petroleum ether (60- 80 ºC) to de fat the material. The defatted material was then extracted with Hexane: ethanol complex to get extract10. The extracts was concentrated for further studies at reduced pressure and temperature in a rotary evaporator and tested for presence of secondary metabolites by different phytochemical tests10. Diiferent concentrations of extract were prepared by dissolving the fine powder in 10% aqueous Dimethylsulfoxide (DMSO) for further study15. |
| |
|
Preliminary Phytochemical Analysis
|
| |
| The Butea monosperma (L) Hexane:ethanol [1:1 ratio] leaf extract was screened for the phytochemical bases using the standard Method10, 11. The phytochemical components analyzed were alkaloids, steroids, starch, proteins, anthraquinone glycosides, saponins, flavonoids, tannins, and cardiac glycosides. |
| |
|
Preparation of bacteria
|
| |
| The bacteria Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Escherichia Coli were purchased from M.T.C.C Institute of Microbial Technology, Chandigarh, India (Invoice No. 9/7/5790). The ability of the various extracts to inhibit growth of clinical bacteria and fungi isolates was determined using the Agar disc diffusion method. Sterile filter paper discs, 11 mm in diameter were impregnated with each extract concentration and dried at 30° C in the static incubator. They were then carefully placed aseptically with a forceps on the surface of the Mueller-Hinton (MH) agar plates12 that were pre inoculated with the 24 hr culture of bacteria and 0.1 ml spore suspension (1 x 105 spores/ml). The control antibiotics disc containing gentamicin (40µg/ml) was placed on each of the inoculated plates of nutrient agar. The plates were left on the bench undisturbed for few minutes, after which the bacterial culture plates were incubated at 37° C for 24 h. The external diameters of visible zones of growth inhibition were measured after incubation13. |
| |
STATISTICAL ANALYSIS
|
| |
| Data collected in the study are expressed as the mean ± standard error of mean (S.E.M.) and statistical analysis was carried out by using one-way analysis of variance (ANOVA) method. P value of less than 0.05 was considered to be statistically significant. All groups were compared with Dimethyl sulfoxide treated control group. |
| |
RESULTS
|
| |
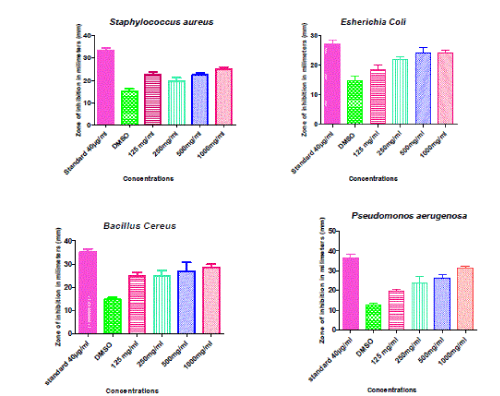
| From table 1 we can conclude that the presence of phytochemicals such as alkaloids, carbohydrates, flavonoids, tannins and phenolics and from table 2 the extract had shown significant Zone of inhibition (millimeter) in concentration dependent manner, 125mg/ml 19.8±1.393 (P<0.05), 250mg/ml 22.6±0.979 (P<0.001), 500mg/ml 22.4±1.122 and 1000mg/ml 22.5±0.86 (P<0.001) in staphylococcus aureus when compared with control group. In case of E.Coli 125mg/ml not shown significant 18.6±1.503, remaining other concentration shown significant with the 250mg/ml 22±0.707 (P<0.01), 500mg/ml 24±2.074 and 24±1.095 (P<0.001) when compared with the control DMSO treated group 14.6±1.72. Incase of B cereus 125 mg/ml 25±1.517 (P<0.01), 250mg/ml 25±2.387 (P<0.01), 500mg/ml 27±3.768 (P<0.01) and 1000mg/ml 28.6±1.288 (P<0.001) extract treated group when compared with control DMSO treated group14.8±0.860. Similarly for P.aerugenosa 125mg/ml shown no significance 19.6±1.03, 250mg/ml 26±1.844 (P<0.01), 500mg/ml 24±3.033 and 1000mg/ml 35.4±1.72 (P<0.001) when compared with control DMSO treated group. |
| |
DISCUSSION
|
| |
| The presence of some of the phytochemical components like saponins, tannins and phenolic compounds have been attributed to the antibacterial activity of the crude drugs observed. Tannins and alkaloids were demonstrated to inhibit the growth of E.coli, P.aeruginosa, B.cereus and S.aureus . The presence of these bioactive components in the crude drugs have been linked to their activities against disease causing microorganisms and also offering the plants themselves protection against infection by pathogenic micro-organisms14. |
| |
| The leaf extract of Butea monosperma (L) Hexane:ethanol [1:1 ratio] is found to contains alkaloids, carbohydrates, flavonoids, tannins and phenolics. The presence of some of the phytochemical components like alkaloids, carbohydrates flavonoids, tannins and phenolics.Compounds have been attributed to the antibacterial activity of the Hexane:ethanol [1:1 ratio] extract of the leaves of Butea monosperma(L) and the different concentrations of extract were found to be effective against some strains of E.Coli, P.aeruginosa, B.cereus and S.aureus in dose dependent manner when compared with Gentamicin which was measured in terms of Zone Of Inhibition (ZOI). |
| |
CONCLUSION
|
| |
| In conclusion Hexane:Ethanolic [1:1] extract of Butea monosperma (L) bark was assessed in this study. The results seem to justify their continued use in the treatment of microbial infections. The inhibition of growth of the test organisms that are known to cause nosocomial infections and displaying multidrug resistance to most antibiotics and nonantibiotic antimicrobial agents justify the continued use of these plants in folk and traditional medical practice. Studies should therefore be done in order to identify the active phytochemical constituents and evaluate their effectiveness in vitro so that they can be synthesized and commercial production begins in earnest. |
| |
 |
| |
ACKNOWLEDGEMENT
|
| |
| Authors are grateful to K.V. Vishnu raju garu chairman Vishnu Institute of Pharmaceutical Education and Research (VIPER), Director of Shri Vishnu educational society, Principal Dr V.H.K.Varma teaching and Non-teaching faculty members for their continuous support while carrying out this research project |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |







