Swathi V1*, Sathish Kumar V2, Abdul Rahaman SK3, Anjana Male4 and Varalakshmi T1
1Department of Pharmacology, Nirmala College of Pharmacy, Atmakur, Guntur, Andhra Pradesh, India
2PharmD, Nirmala College of Pharmacy, Atmakur, Guntur, Andhra Pradesh, India
3Department of Chemistry, Nirmala College of Pharmacy, Atmakur, Guntur, Andhra Pradesh, India
4Department of Phytochemistry, Nirmala College of Pharmacy, Atmakur, Guntur, Andhra Pradesh, India
*Corresponding Author:
Swathi V
Department of Pharmacology, Nirmala College of Pharmacy
Atmakur, Guntur, Andhra Pradesh, India
Tel: +919493388790
E-mail: swathi.pharmabud@gmail.com
Received date: July 29, 2017; Accepted date: August 30, 2017; Published date: Setember 01, 2017
Citation: Swathi V, Kumar SV, Rahaman ASK, Male A, Varalakshmi T (2017) In-vivo Screening of Analgesic and Antiulcer Activity on Carum carvi Seeds. Int J Drug Dev & Res 9: 18-24
Keywords
Carum carvi; Antiulcer activity; Aspirin; Analgesic
Introduction
An analgesic or painkiller is any member of the group of drugs used to achieve analgesia, relief from pain. A number of herbal drugs are used to treat Analgesia. Several epidemiological studies from different countries have reported widely varying prevalence rates for chronic pain, ranging from 12 to 80% of the population [1-6]. It becomes more common as people approach death. A study of 4,703 patients found that 26% had pain in the last two years of life, increasing to 46% in the last month [2]. The intensity of chronic pain was higher for girls, and girls' reports of chronic pain increased markedly between ages 12 and 14 [3]. Perception gives information on the pain's location, intensity, and something about its nature. The various conscious and unconscious responses to both sensation and perception, including the emotional response, add further definition to the overall concept of pain [4]. Pain perception also varies depending on the location of the pain. The kinds of stimuli that cause a pain response on the skin include pricking, cutting, crushing, burning, and freezing. These same stimuli would not generate much of a response in the intestine. Intestinal pain arises from stimuli such as swelling, inflammation, and distension [4]. Pain is the main reason for visiting the emergency department in more than 50% of cases and is present in 30% of family practice visits [5]. Several epidemiological studies from different countries have reported widely varying prevalence rates for chronic pain, ranging from 12 to 80% of the population [6]. It becomes more common as people approach death. A study of 4,703 patients found that 26% had pain in the last two years of life, increasing to 46% in the last month [7]. The intensity of chronic pain was higher for girls, and girls' reports of chronic pain increased markedly between ages 12 and 14 [8]. The success of NSAIDs in treating various inflammatory conditions such as rheumatoid arthritis (RA) and osteoarthritis (OA) validated inhibition of the enzyme prostaglandin H synthase (PGHS) or cyclooxygenase (COX) as a highly suitable target in anti-inflammatory therapies [9,10]. The following NSAIDs are derived from fenamic acid. Which is a derivative of anthranilic acid [11], which in turn is a nitrogen isostere of salicylic acid, which is the active metabolite aspirin [11]. The first purified preparation of COX enzyme was reported in 1976 [12]. More than a decade later COX was cloned in 1988 [13-15]. In the 1990’s an inducible isoform now called COX-2 was discovered [16-18]. Inflammation (Latin, inflammation) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants [19-32].
Caraway (Carum carvi), also known as meridian fennel, [19-22] or Persian cumin, [31,33] is a biennial plant in the family Apiaceae, [23] native to western Asia, Europe, and northern Africa. it occurs in India to a limited extent in the temperate regions of the western Himalayas, Jammu and Kashmir and in chakratha hills of utter Pradesh. however, polyploid variants (with four haploid sets=4 n) of this plant that was found to be perennial. Finland supplies approximately 28% (2011) of the world's caraway production [24]. Caraway seeds are a rich source of dietary fiber. 100 g seeds provide 38 g of fiber,100% of daily recommended intake of fiber. The fruits, usually used whole, have a pungent, anise -like flavor and aroma that comes from essential oils, mostly carvone, limonene and anethole [25,26]. It is used in caraway seed cake, and it is frequently added to sauerkraut [27-30]. The roots may be cooked as a vegetable like parsnips or carrots. Additionally, the leaves are sometimes consumed as herbs, either raw, dried, or cooked, similar to parsley [31-38].
Materials and Methods
Extraction and preparation of test sample
The seeds of caraway were collected, dried and grinded into fine powder. 250 grams of caraway powder was weighed and extracted by using two types of solvents. Extraction was carried out by using 500 ml of aqueous and hydroalcohol respectively by using maceration process. Until the complete extraction takes place. The extract obtained was filtered by using vaccum filtration and further, concentrated by using evaporation. The extract was further dried under air.
Albino Wistar strain rats obtained from Mahaveera enterprises, peerzadiguda, ghatkesar mandal, R.R. Dist-39. weighing 150-250 gm of either sex was used in this study. Animals were acclimatized to laboratory conditions before testing e.g., room temperature of 25 ± 1°C, relative humidity 45-55% and a 12:12 h light dark cycle.
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) and protocol approval No. is 011/ IAEC/Ncpa/Researchproposal/2015/16. The committee for purpose of control and supervision of experiments on animals (CPCSEA) Reg. No. is 1629/po/a/12/CPCSEA. All the experimental procedures were carried out according to the proper guidelines of CPCSEA.
Analgesic studies
Tail immersion method: In present study analgesia was assessed according to the method of Luiz et al. The animals fasted for 12 hours before the procedure was started. A total of six rats was grouped into four groups. One group treated with distilled water serves as control. The second group was treated Diclofenac sodium which acts as as standard. Other two groups were given test drugs i.e, caraway extract of 100 mg/kg and 200 mg/kg. The drug is administered through oral route by using oral feeding needle. They were held in position in a suitable restrainer with a tail extending out. 3-4 cms of the tail were marked and immersed in water bath thermostatically maintained at 55°C. The withdrawal time of tail from hot water (in seconds) was noted as reaction time or tail flick latency. The maximum cutoff time for immersion was 15 seconds to avoid the injuries of tissues of the tail. The initial reading was taken immediately before administration of the test and standard drugs and then 15,30,45,60 and 75 minutes after the administration.
Anti-ulcer screening method: Antiulcer activity was evaluated by an aspirin-induced ulcer in Wistar rats [8].
Aspirin-induced gastric ulcer model: The animals fasted for 24 hours before the experiment. Animals were divided into 4 groups each containing 6 animals. Group 1 served as a positive control and received omeprazole at the dose of 30 mg/kg body weight, group 2 served as a negative control received aspirin at the dose of 500 mg/kg body weight. Group 3 received the aqueous extract of caraway seeds at the dose of 500 mg/kg body weight and group 4 received the hydroalcoholic extract of caraway seeds at the dose of 500 mg/kg body weight. After one hour of extract administration, aspirin at a dose of 500 mg/kg body weight was administered orally to all four groups. Then after 6 hours animals were euthanized with an excess of chloroform and the stomach was excised and cut along the greater curvature, washed and the inner surface was examined for ulceration. Ulcer index and % ulcer protection were calculated by using the methods described in (Tables 1-5). The ulcer index was then calculated by adding the total number of ulcers per stomach and total severity of ulcers per stomach.
| Time in minutes |
At 0 min
Time in sec |
At 15 min
Time in sec |
At 30 min
Time in sec |
At 45 min
Time in sec |
At 60 min
Time in sec |
At 75 min
Time in sec |
| Control |
2.5 ± 0.02 |
2.5 ± 0.02 |
2.55 ± 0.05 |
2.6 ± 0.05 |
2.5 ± 0.02 |
2.5 ± 0.02 |
| Standard |
2.5 ± 0.05*** |
6.2 ± 0.15*** |
8.4 ± 0.2*** |
12 ± 0.4*** |
9 ± 0.5*** |
5 ± 0.5*** |
| Aqueous 100mg |
2.6 ± 0.11ns |
5.6 ± 0.04** |
8.1 ± 0.07** |
10.5 ± 0.1** |
6.5 ± 0.09** |
5.5 ± 0.1** |
| Aqueous 200mg |
2.5 ± 0.1ns |
6.3 ± 0.09ns |
8.4 ± 0.1ns |
12.5 ± 0.1** |
10.3 ± 0.16** |
8 ± 0.08** |
Values are mean ± SD, n=6 rats in each group. Significant values calculated by dunnet’s multiple comparision test. Here p values, ***p: <0.001-Highly significant, **P:<0.01-Significant, p value: >0.05-Non-significant
Table 1: Results of aqueous extract of Carum carvi seeds by using tail immersion method.
| Time in min |
At 0 min
Time in sec |
At 15 min
Time in sec |
At 30 min
Time in sec |
At 45 min
Time in sec |
At 60 min
Time in sec |
At 75 min
Time in sec |
| Control |
2.5 ± 0.2 |
2.5 ± 0.2 |
2.5 ± 0.5 |
2.6 ± 0.05 |
2.5 ± 0.02 |
2.5 ± 0.02 |
| Standard |
2.5 ± 0.05*** |
6.2 ± 0.15*** |
8.4 ± 0.2*** |
12 ± 0.4*** |
9 ± 0.5*** |
5 ± 0.5*** |
| Hydro alcoholic 100mg |
2.5 ± 0.07ns |
4.4 ± 0.23** |
6.4 ± 0.15** |
10.2 ± 0.14** |
6.2 ± 0.20** |
5.1 ± 0.14ns |
| Hydro alcoholic 200mg |
2.5 ± 0.08ns |
5.4 ± 0.09** |
7.4 ± 0.15** |
11.2 ± 0.17** |
9 ± 0.37ns |
5 ± 0.1ns |
Values are mean ± SD, n=6 rats in each group. Significant values calculated by dunnet’s multiple comparision test. Here p values, ***p: <0.001-Highly significant, **P:<0.01-Significant, p value: >0.05-Non-significant
Table 2: Results of hydro alcoholic extract of Carum carvi seeds by using tail immersion method.
| Group |
Volume of Centrifuge(ml) |
Volume of NaOH Consumed(ml) |
Total Acidity
(mEq/L) |
Ulcer Index |
% Ulcer Protection |
| Control |
1 ± 0.36 |
2.5ml ± 0.72 |
25 ± 2.56 |
6 ± 1.05 |
- |
| Standard |
0.8 ± 0.34 |
1 ± 0.36 |
10 ± 0.45*** |
1.5 ± 0.51*** |
75 ± 3.57 |
| Hydro alcoholic 100 mg |
0.7 ± 0.37 |
2.1 ± 0.4 |
21 ± 1.96** |
5 ± 0.7** |
16.7 ± 0.37 |
| Hydro alcoholic 200 mg |
0.4 ± 0.58 |
1 ± 0.36 |
10 ± 0.47ns |
2 ± 0.58** |
66.7 ± 1.09 |
Values are mean ± SD, n=6 rats in each group. Significant values calculated by dunnet’s multiple comparision test. Here p values ***p: <0.001-Highly significant, **P: <0.01-Significant, p value: >0.05- Nonsignificant
Table 3: Results of anti-ulcer activity using hydro-alcoholic extract of Carum carvi seeds against aspirin-induced gastric ulcers.
| Group |
Volume of Centrifuge (ml) |
Volume of NaOH Consumed (ml) |
Total Acidity (mEq/L) |
Ulcer Index |
% Ulcer Protection |
| Control |
1 ± 0.36 |
2.5 ± 0.72 |
25 ± 2.56 |
6 ± 1.05 |
- |
| Standard |
0.8 ± 0.34 |
1 ± 0.36 |
10 ± 0.45*** |
1.5 ± 0.51*** |
75 ± 3.57 |
| Aqueous extract 100 mg |
0.8 ± 0.36 |
1.8 ± 0.53 |
18 ± 0.58** |
5.5 ± 0.52** |
8.3 ± 0.38 |
| Aqueous extract 200 mg |
0.4 ± 0.58 |
1.3 ± 0.36 |
13 ± 0.55** |
3 ± 0.48** |
50 ± 0.51 |
Values are mean ± SD, n=6 rats in each group. Significant values calculated by dunnet’s multiple comparision test. Here p values ***p: <0.001-Highly significant, **P: <0.01-Significant, p value: >0.05- Nonsignificant
Table 4: Results of anti-ulcer activity using aqueous extract of Carum carvi seeds against aspirin-induced gastric ulcers.
| S. No |
Name of the Experiment |
Hydro alcoholic extract |
Hydro extract |
| 1 |
Detection of Alkaloids: |
- |
- |
| 2 |
Test for Glycosides: |
- |
- |
| 3 |
Test for cardiac glycosides: |
- |
- |
| 4 |
Tests for deoxy sugars: |
- |
- |
| 5 |
Tests for anthraquinone glycosides: |
- |
- |
| 6 |
Tests for cyanogenetic glycosides |
- |
- |
| 7 |
Test for saponin glycosides: |
- |
- |
| 8 |
Test for coumarin glycosides |
- |
- |
| 9 |
Test for carbohydrates: |
- |
- |
| 10 |
Test for non-reducing sugars: |
- |
- |
Table 5: Table showing results of phytochemical screening.
Ulcer index
Procedure: Animals in the group of aspirin induced ulcer were starved for 24 hours. The pylorus is ligated and the abdominal wall is closed with sutures.3 hours after pyloric ligation, the animals were sacrificed. The abdomen was opened and a ligature was placed around the esophagus. The stomach was removed and fixed on a cork plate and the number of and severity of ulcers was registered with a stereomicroscope using the following scores.
Ulcer index was calculated using following formula:

Determination of total acidity and free acidity
Procedure: 0.1 mL of gastric juice specimen was transferred in a porcelain evaporating dish. 1-2 drops of Topfer’s reagent is added. A colour change was observed; a bright red colour appears if free hydrochloric acid is present. 1-2 drops of phenolphthalein were added to the gastric juice with Topfer’s reagent. Titrated with 0.01 N NaOH from a burette, mixing was done after each addition until the last trace of red colour disappeared and was replaced by a canary yellow colour. The numbers of milliliters of NaOH used was read from the burette. This represents the amount of free hydrochloric acid. The titration was continued until the red colour of phenolphthalein appeared (deep pink), titrated to the point at which the further addition of alkali did not deepen the colour. Reading was taken (mL NaOH) for total acidity.
Calculation: Y=mL of 0.1N NaOH × 10
where, Y=Total acidity (mEq/L); Acidity=Volume of NaOH × Normality × 100 mEq/litre
Acid volume
Procedure: Under ether anesthesia, a midline incision is made. Herbal extract was given to rats as per dosages and after a span of 1 hour aspirin was given at a dose of 500 mg/kg body weight. 3 hours after ingestion of aspirin, the animals were sacrificed. The abdomen was opened and a ligature was placed around the esophagus. The stomach was removed and the contents were drained into graduated centrifuge tube through a small nick along the greater curvature. The volume of the juice was measured.
Induction of gastric ulcer: The animals were fasted for 36 hours in separate cages with raised wide wire mesh to avoid coprophagia (Basso et al.), but with water given ad libitum. After 4 hours under light anesthesia by ketamine, animals were killed, stomach was removed and opened on greater curvature and examined for ulceration. Evaluation of the degree of ulceration was expressed in terms of ulcer score which is calculated by dividing the total number of ulcers in each group by a number of rats in that group (Robert et al.). The degree of ulceration was also expressed as ulcer index and calculated by multiplying ulcer score × 100 (Radwan et al.).
Preventive index was calculated according to the method:
Preventive index=U.I control-U.I. treated × 100 U.I. treated group
% of ulceration=Number of ulcerated rat-Number of non-ulcerated rats × 100/Number of rat in group
Statistical analysis: The values Mean ± SEM are calculated for each parameter. For determining the significant intergroup difference each parameter was analyzed separately and one-way analysis of variance [13] (Gennaro) was carried out and the individual comparisons of the group mean values were done using Dunnet’s test [14] (Dunnet).
Results
Results of this study are presented in Tables 1-4 and Figures 1-5.
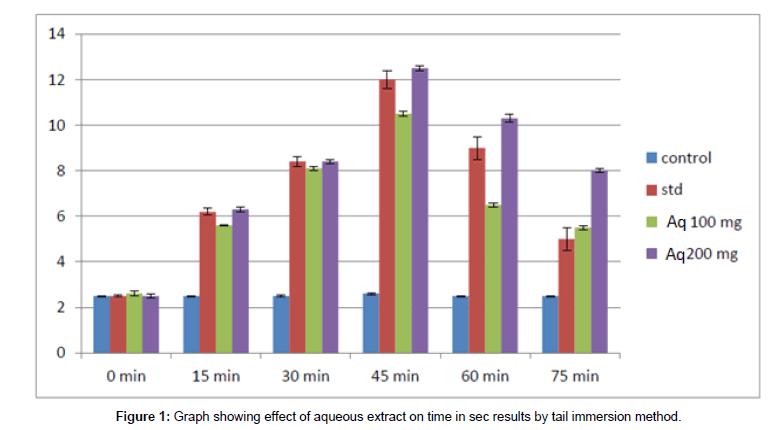
Figure 1: Graph showing effect of aqueous extract on time in sec results by tail immersion method.
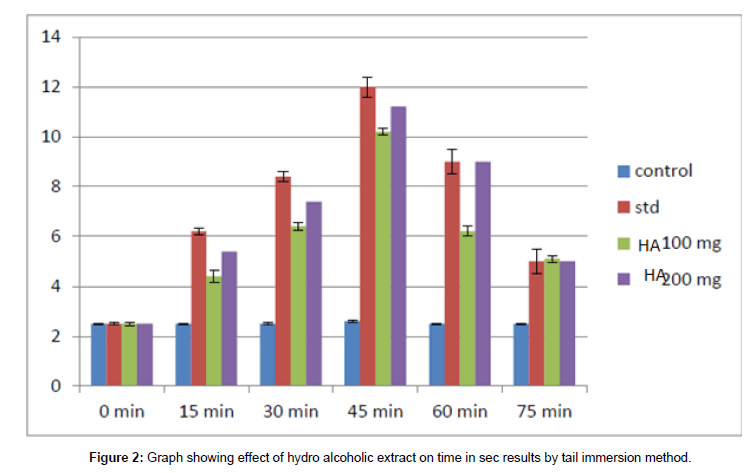
Figure 2: Graph showing effect of hydro alcoholic extract on time in sec results by tail immersion method.
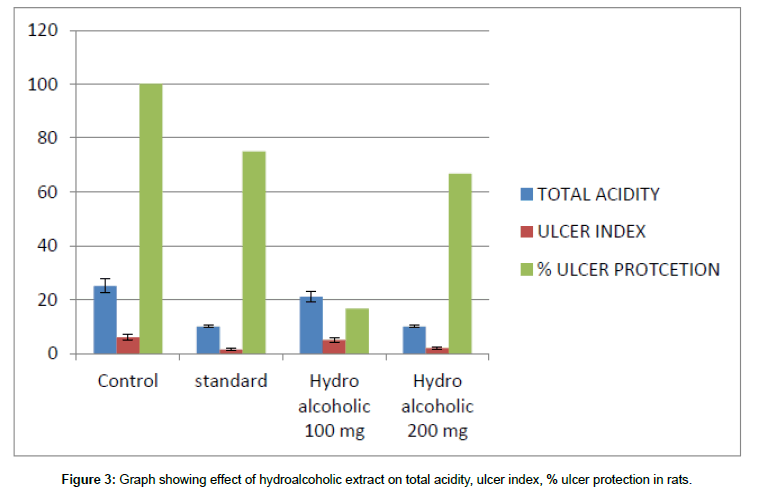
Figure 3: Graph showing effect of hydroalcoholic extract on total acidity, ulcer index, % ulcer protection in rats.
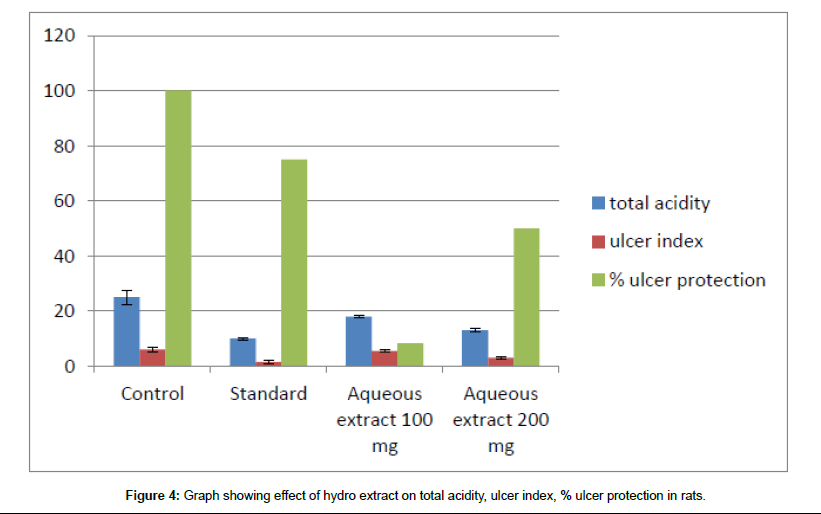
Figure 4: Graph showing effect of hydro extract on total acidity, ulcer index, % ulcer protection in rats.
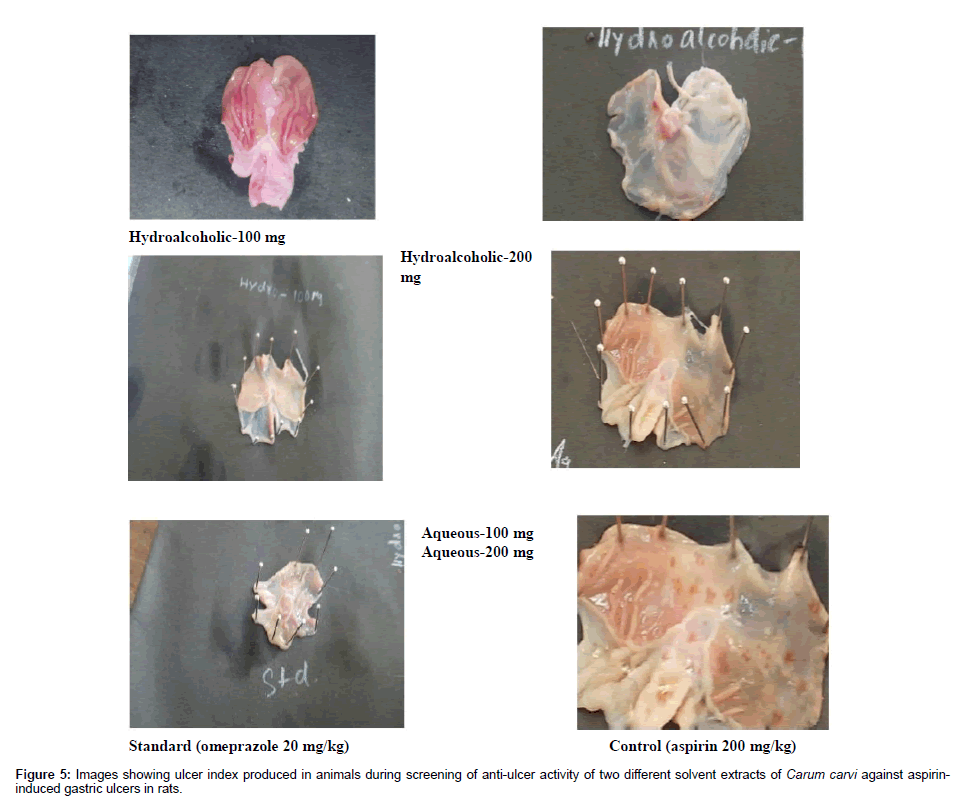
Figure 5: Images showing ulcer index produced in animals during screening of anti-ulcer activity of two different solvent extracts of Carum carvi against aspirininduced gastric ulcers in rats.
Phytochemical screening results
Phytochemical screening results are presented in Table 5.
Discussion
Analgesic studies
The time taken in seconds for the rat to withstand its tail in hot water at 55°C was noted. In control animals, without any drug, almost all rats withstand for approximately 2.5 sec at varying time intervals. The time taken for the standard group i.e., group of rats treated with standard drug Diclofenac sodium the time in seconds for the rat to withdrawal its tail from hot water is increased with increase in time. At a maximum of 45 min after ingestion of standard drug the rat could withstand for 12 secs showing the analgesic effect. In comparison with the time in second values at increasing time in test groups treated with hydro (or) aqueous extract 100 mg and 200 mg and also in the group treated with a hydroalcoholic extract of 100 mg and 200 mg, the values are also increasing.
Determination of ulcer index
The mean ulcer index value in control group is 6 and for standard drug Omeprazole. The mean ulcer index for test groups treated with hydroalcoholic extract 100 mg and 200 mg is 5 ± 0.7 and 2 ± 0.58 and in group treated with hydro/aqueous extract at dose of 100 mg and 200 mg is 5.5 ± 0.52 and 3 ± 0.48.
Determination of percentage ulcer protection
In the control group, almost all animals are positive for an ulcer. For the group treated with standard drug Omeprazole 75% of the population in the group is protected from an ulcer. In the case of groups treated with a hydroalcoholic extract of 100 mg and 200 mg dose almost 16.7% and 66.7% protection is observed in a population and in the case of the group treated with aqueous extract at a dose of 100 mg and 200 mg 8.3% and 50% protection is observed.
Phytochemical screening
Proteins, tannins, phenolic compounds and flavonoids are present. Alkaloids, glycosides, carbohydrates, non-reducing sugars, steroids, aminoacids, saponins, volatile oils are absent in the screening of phytochemicals.
Conclusion
Review literature supported the use of seeds of Carum carvi for Analgesic and Anti-Ulcer Activity. Extraction using aqueous and hydroalcoholic solvents was done. Screening of Analgesic activity is done by using tail immersion method at a dose of 100 mg and 200 mg by using two extracts. In comparison with the standard drug, the results of hydroalcoholic extract at 100 mg dose showed good analgesic activity compared with a standard drug. Anti-Ulcer activity is screened by using Aspirin-induced ulcer at doses of 100 mg and 200 mg. At a dose of 200 mg of hydroalcoholic extract, the percentage of ulcer protection in test group is almost similar with ulcer protection with a standard drug. It decreases the total acidity and ulcer index. Finally, we conclude that hydroalcoholic extract at a dose of 100 and 200 mg shows good Analgesic and Anti-Ulcer Activity. The drug was selected for a future investigation involving column chromatographic separation of extracts and to isolate and identify the actual chemical constituent responsible for the activity.
20406
References
- Lewis C, Short C (1879) A Latin Dictionary, by Charlton T Lewis and Charles Short. Oxford/Clarendon Press.
- Smith AK, Cenzer IS, Knight SJ, Puntillo KA, Widera E, et al. (2010) The epidemiology of pain during the last 2 years of life. Annals of Internal Medicine 153: 563-569.
- Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, Van Suijlekom-Smit LW, et al. (2000) Pain in children and adolescents: A Common Experience. Pain 87: 51-58.
- Hasselström J, Liu-Palmgren J, Rasjö-Wrååk G (2002) Prevalence of pain in general practice. Eur J Pain 6: 375-385.
- Abu-Saad Huijer H (2010) Chronic Pain: A Review. J Med Liban 58: 21-27.
- Vane JR (1976) The mode of action of aspirin and similar compounds. J Allergy Clin Immunol 58: 691-712.
- Moncada S, Ferreira SH, Vane JR (1973) Prostaglandins, aspirin-like drugs and the edema of inflammation. Nature 246: 217-219.
- Sriram D, Yogeeswari P (2010) Medicinal Chemistry. Pearson Education India. 2nd edn.
- Miyamoto T, Ogino M, Yamamoto S, Hayaishin O (1976) Purification of prostaglandin endoperoxide synthetase from bovine vesiculargl and microsomes. J Biol Chem 259: 2629-2636.
- DeWitt DL, Smith WL (1988) Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci 85: 1412-1416.
- Merlie JP, Fagan D, Mudd J, Needleman P (1988) Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem 263: 3550-3553.
- Yokoyama C, Takai T, Tanabe T (1988) Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett 231: 347-351.
- Masferrer JL, Zweifel BS, Seibert K, Needleman P (1990) Selective regulation of cellular Cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest 86: 1375-1379.
- Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL (1991) Expression of amitogen-responsive gene encoding prostaglandin synthase is regulated by MRNA Splicing. Proc Natl Acad Sci USA 88: 2692-2696.
- Kujubu DA, Herschman HR (1992) Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem 267: 7991-7994.
- Wikipedia (2016) Caraway. Available at: https://en.wikipedia.org/wiki/Caraway
- Buzzle (2016) Anise seedsubstitute. Available at: https://www.buzzle.com/articles/anise-seed-substitute.html
- Kerala Recipes (2013) English Malayalam spice names. Available at: https://malayali.me/english-malayalam-spice-names
- Word Crops Database (2016) Caraway. Available at: https://world-crops.com/caraway-seeds/
- USDA Plants Classification Report (2017) Apiaceae. Available at: https://plants.usda.gov/java/ClassificationServlet?source=profile&symbol=Apiaceae&display=31
- Goodnews from Finland. Available at: https://www.goodnewsfinland.com/archive/news/finland-a-global-leader-in-caraway-exports
- https://www.aromaticscience.com/chemical-composition-and-antiulcerogenic-activity-of-the-volatile-oil-from-carum-carvi/
- María DL, María JJ, María JPV (2008) Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. Journal of Stored Products Research44: 273-278.
- Cooks.com Recipe Search (2013) German Sauerkraut Caraway. Available at: https://www.cooks.com/recipe/p61h91sm/german-sauerkraut-caraway.html
- Low-cholesterol.Food.com (2013) Sauerkraut with Caraway Recipe. Available at: https://www.food.com/recipe/sauerkraut-with-caraway-206206
- Food Network (2013) Fancified sauerkraut Recipe. Emeril lagasse: Recipes. Available at: https://www.cookingindex.com/recipes/33216/fancified-sauerkraut.htm
- Allrecipes (2013) Slow Cooker Kielbasa Stew Recipe. Available at: https://allrecipes.com/recipe/25676/slow-cooker-kielbasa-stew/
- Kumar JP, Shankar NB (2009) Analgesic activity of mollugo pentaphyllaLinn. by the tail immersion method. Asian J Pharm Clin Res 2: 61-63.
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE, Nielsen A, et al. (2007) Chronic Inflammation: Importance of NOD2 And NALP3 In Interleukin-1 beta Generation. Clin Exp Immunol 147: 061127015327006.
- Pharmacompass (2017) Plant Name: Meridian. Available at: https://www.pharmacompass.com/active-pharmaceutical-ingredients/meridian-fennel
- Epicurious (2013) sauerkraut with apple and caraway recipe at. Available at: https://www.epicurious.com/recipes/food/views/sauerkraut-with-apple-and-caraway-13352
- Yokoyama C, Takai T, Tanabe T (1988) Primary structure of sheep prostaglandin endoperoxides synthase deduced from cDNA sequence. FEBS Lett 231: 347-351.
- Picot D, Loll PJ, Garavito RM (1994) The x-ray crystal structure of the membrane protein prostaglandin H2 synthase 1. Nature 367: 243-249.
- Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Ann Rev Biochem 69: 145-182.
- Smith WL, DeWitt DL (1996) Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62: 167-215.
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, et al. (1997) Multiple female reproductive failures incyclooxygenase-2 deficient mice. Cell 91: 197208.
- Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, et al. (1999) Angiotensin II attenuates renal corticalcyclooxygenase-2 expression. J Clin Invest 103: 953-961.












