Keywords
Sepsis; Procalcitonin (PCT); C-reactive protein (CRP); Blood culture; Newborns
Introduction
Sepsis is a systemic response to infection by microbial organisms and it is a leading cause of major cause of mortality and morbidity in newborns with the highest incidence occurring among newborns of very low birth weight and gestation [1,2]. Without timely treatment, sepsis can rapidly lead to tissue damage, organ failure, and death in newborns. Preterm newborns are at higher risk for sepsis than term newborns, as they tend to require more invasive procedures than term newborns [3,4]. In the new progress in assessing the risk of developing sepsis, severe sepsis or septic shock is the importance of biochemical monitoring. Unfortunately, the leading factor in morbidity and mortality in the postnatal period is still bacterial inflammation [5]. Newborns with clear signs of sepsis present with features of infection and clinical manifestations of inflammation.Severe sepsis is the development of hypoperfusion with organic dysfunction in a septic patient. Septic shock is hypoperfusion with multiorgan failure and persistent hypotension [6,7]. Mortality varies up to 16% in newborns with sepsis and 40-60% in newborns with septic shock. The research of new biochemical markersenabling a precocious identification of neonates at risk of neonatal diseases, allowing a close monitoring of thedisease and providing information about prognosis,represents a strategic objective of several currentresearches [8].
Procalcitonin is a peptide precursor to the hormone calcitonin, the latter being involved with calcium homeostasis. It is usually synthesized in the C cells of the thyroid gland and neuroendocrine cells of the lung and the intestine. It occurs when preprocalcitonin is broken down intracellularly under the action of proteolytic enzymes into the active hormone [9,10]. In structure it is a protein made up of 116 amino acids with a molecular weight of 13 kDa [11,12]. Procalcitonin has a halflife of 22 to 26 hours and can be detected within 4 hours. The peak occurs between 12-48 hours. Under normal physiological conditions in humans, procalcitonin levels are <0.5 ng/ml. In addition to thyroid cells, PCT is synthesized in other extrathyroid tissues and its concentration can be increased up to 100,000 times in bacterial infection as well as in systemic inflammatory response syndrome (SIRS). PCT synthesis is activated by a number of pro-inflammatory cytokines released in the presence of bacterial toxins, leading to an increase in PCT concentration >0.5 ng/ml. However, procalcitonin can be elevated in diseases other than sepsis, such as autoimmune diseases, heart attack, pancreatitis, trauma, burns, and surgery. PCT in clinical practice is used as a biomarker to distinguish bacterial from viral sepsis, as well as non-infectious systemic inflammatory response syndrome (SIRS) [13,14]. Compared to IL-2, IL-6, IL-8, CRP, and TNF-alpha, procalcitonin has the highest sensitivity (90%) and specificity (91%) for differentiating newborns with systemic inflammatory response syndrome from those with sepsis [15]. Procalcitonin kinetics have also been shown to determine the severity of infection and the efficacy of antimicrobial therapy.
Lactates are metabolites of glucose and are created in the human body whenever there is a lack of oxygen in the tissues. Lactate is produced by most tissues in the human body, with the highest level of production found in muscle. Under normal conditions, lactate is rapidly cleared by the liver, with a small amount of additional clearance by the kidneys. Lactate exists in 2 isomers: l-lactate and d-lactate. Current lactate measurements include only L lactate (the primary isomer produced in humans), while D lactate is produced by bacteria in the human colon in the presence of large amounts of carbohydrates that are not absorbed in the gut. There is a constant concentration of lactates in the blood 1-1.5 mmol/L. Blood lactate concentrations reflect the balance between lactate production and clearance. But in critically newborns suffering from hypoperfusion or in newborns with shock, lactate levels rise above 2 mmol/L, and if the level rises above 4 mmol/L, it is an indication for admission to the intensive care unit (therapy) [16,17]. Lactate concentrations have high prognostic value as well as mortality in patients with septic shock. Thus, in patients who are susceptible to sepsis, measurement of lactate levels provides information on the monitoring and severity of the condition and disease progression [18].
Early detection of this disease is required in order to initiate therapy in a timely manner and reduce the mortality rate. Procalcitonin and lactates are used as biomarkers for early detection of sepsis.
Methods
In a prospective study, 100 newborns with proven sepsis were included from period January 2019 year till August 2020 year. Diagnosis of sepsis in newborns diagnosed according to standard protocols for diagnosis of disease. The clinical criteria taken as indicative of sepsis in newborns were: respiratory distress, lethargy, apnea,tachypnea, bradycardia, seizures, poor perfusion, lethargy, feeding intolerance, temperature instability, low birth weight, gestational age, gender, preterm newborns.
Laboratory examination were analyzedin the Clinical Laboratory at the University Children Hospital-Skopje. Sample for blood culture, PCT and blood gas-lactate were taken the first at the admission, the second on 3 day and the third 7 day Blood culture media were incubated at 37°C for 5 days in BactAlert 3D 360. Positive blood culture were proven with the new multiplex polymerase chain reaction–based rapid diagnostic test (BioFire FilmAray Blood Culture Identification). Procalcitonin was determined by immunoassay: patented ELFA (Enzyme-linked fluorescent assay) technology, automated Vidas Biomerieux immunoassay (ng/ml) . Blood gas-lactate were determined with Rapid point 500 Siemens.
Statistic method
SPSS program was used for statistical analysis,to compare means of the variables, one-way ANOVA test. Categorical variables between groups were analyzed using Chi-square test.Results were presented as percent (%), mean, standard deviation (SD), median, and minimum-maximum (min-max). A P-value<0.05 was considered as significant.
Results
In the study was designed as a prospective, we included 100 (M:F=59:41) newborns with proven sepsis hospitalized in the Intensive Care Unit at the University Children Hospital-Skopje in period of January 2019 year till August 2020 year. The newborns with proven sepsis have been divided into two groups. The I group included 50 proven sepsis preterm newborns with positive blood culture and II group included 50 proven sepsis term newborns (Figure 1).
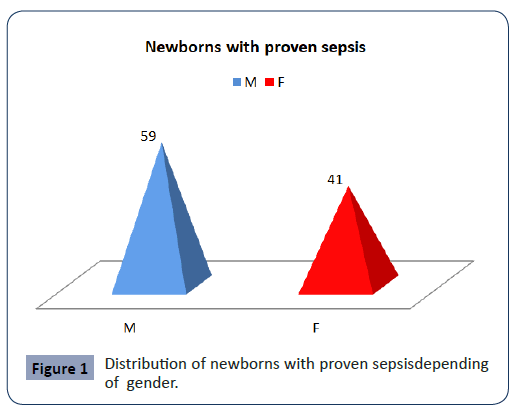
Figure 1: Distribution of newborns with proven sepsisdepending of gender.
The mean gestational age of newborns with proven sepsis was 37.31 ± 3.7 weeks The mean birth weight of newborns with proven sepsis was 2936.5 ± 725.6 grams.
The newborns with proven sepsis in 51 (49%) the cause was ± RDS, in 37 the cause was Asphyxia, in 14 was other comorbidities (Figure 2).
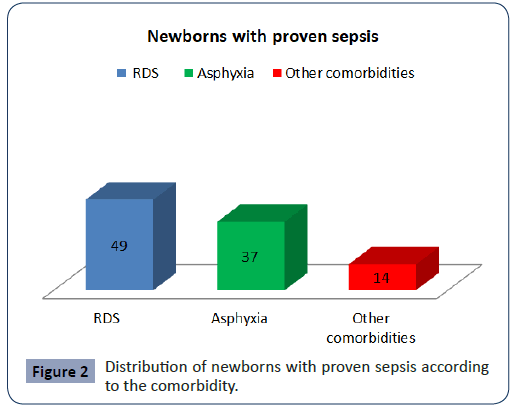
Figure 2: Distribution of newborns with proven sepsis according to the comorbidity.
We isolated fifty seven newborns who had two or three bacteria at the same time. The identified bacteria included Staphylococcus aureus (n=61) mecA, Streptococcus (n=6), Acinetobacter baumannii (n=16), Serratia marcescens (n=8) and Entrobacteriaceae (n=32), Candida albicans (n=1) Candida parapsilosis (n=1).
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in preterm newborns with proven sepsis p<0.001 (Figure 3). The highest average values (44.37 ± 53.59) were measured during admission with a subsequent sharp jump. After the second measurement at 3 day, the average values of PCT slowly decreased (28.5 ± 36.19), so that after the third measurement on7 day they slowly began to normalize (9.76 ± 15.28).
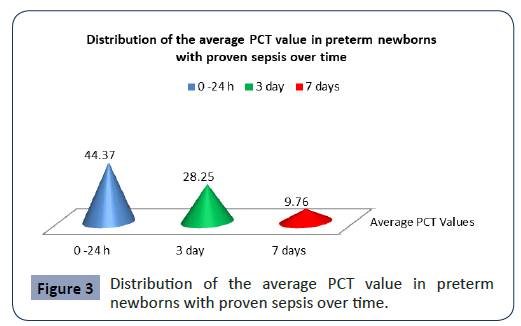
Figure 3: Distribution of the average PCT value in preterm newborns with proven sepsis over time.
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in newborns with proven sepsis with asphyxia p<0.001 (Figure 4). The highest average values (41.37 ± 62.50) were measured at newborns with proven sepsis with asphyxia during admission with a subsequent sharp jump compared with newborns with proven sepsis with RDS and other comorbidities (Figure 4). After the second measurement at 3 day, the average values of PCT at newborns with proven sepsis with asphyxia slowly decreased (36.95 ± 41.31), so that after the third measurement on7 day at newborns with proven sepsis with asphyxia they slowly began to normalize (29.35 ± 28.11).
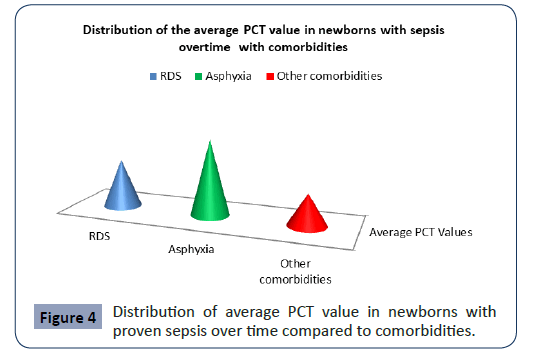
Figure 4: Distribution of average PCT value in newborns with proven sepsis over time compared to comorbidities.
Statistical analysis confirmed significantly different values of Lactate in the analyzed time period in preterm newborns with proven sepsis p<0.001 (Figure 5). The highest average values (3.19 ± 1.19) were measured during admission with a subsequent sharp jump. After the second measurement at 3 day, the average values of PCT slowly decreased (2.05 ± 1.09), so that after the third measurement on 7 day they slowly began to normalize (1.76 ± 1.18).
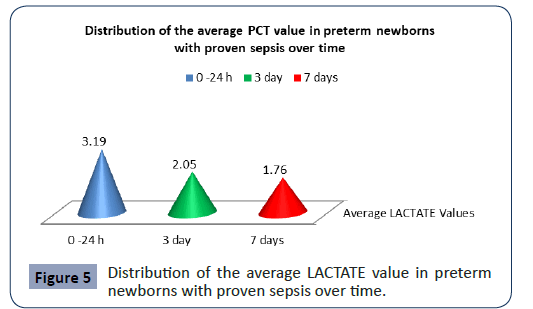
Figure 5: Distribution of the average LACTATE value in preterm newborns with proven sepsis over time.
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in newborns with proven sepsis with asphyxia p<0.001 (Figure 6). The highest average values (3.11 ± 1.02) were measured at newborns with proven sepsis with asphyxia during admission with a subsequent sharp jump compared with newborns with proven sepsis with RDS and other comorbidities. After the second measurement at 3 day, the average values of PCT at newborns with proven sepsis with asphyxia slowly decreased (2.5 ± 1.05), so that after the third measurement on 7 day at newborns with proven sepsis with asphyxia they slowly began to normalize (1,5.15 ± 1.06).
Figure 6: Distribution of average LACTATE value in newborns with proven sepsis over time compared to comorbidities.
Discussion
At the newborns sepsis is a life-threatening condition and still represents an important cause of mortality and morbidity. For pediatrician early identification of infections is still a challenge. The aetiology of sepsis is not always clear and an organism that initiates disease in one newborns may not in another [19,20]. Procalcitonin is an important parameter for early differentiation of bacterial from nonbacterial systemic disease by determining the severity of the disease. Procalcitonin has shown inconsistencies in predicting mortality in patients with sepsis [21]. Therefore, on the other hand, lactates are a strong predictor of mortality and severity of the disease. Lactates are unable to distinguish between infectious or non-infectious etiology and do not provide information on the effectiveness of antibiotic therapy.
Also, many studies have shown that in the early stages, procalcitonin values are higher in chronic diseases such as congestive heart failure, RDS, asphyxia, chronic kidney disease, and this should be taken into account when making a clinical assessment using this biomarker [22]. In septic conditions, the value of procalcitonin may be far higher and procalcitonin is more specific in bacterial infections than other acute phase reactants. An ideal biomarker for sepsis should have high sensitivity and specificity with early phase elevation, low cost and quick result.The diagnostic performance of PCT in numerous studies from literature has suggested PCT to be useful marker in diagnosis of sepsis [23,24]. We examined PCT in newborns with proven sepsis in our study. Statistical analysis confirmed significantly different values of PCT in the analyzed time period in preterm newborns with proven sepsis p<0.001. The highest average values (44.37 ± 53.59) were measured during admission with a subsequent sharp jump. Statistical analysis confirmed significantly different values of PCT in the analyzed time period in newborns with proven sepsis with asphyxia p<0.001 [25,26]. The highest average values (41.37 ± 62.50 were measured at newborns with proven sepsis with asphyxia during admission with a subsequent sharp jump compared with newborns with proven sepsis with RDS and other comorbidities. Lactates are the best marker for tissue perfusion. They are elevated under conditions of anaerobic metabolism, when in fact the demand for oxygen exceeds the supply. This occurs due to decreased arterial oxygen content (hypoxemia), maldistribution of flow, decreased perfusion pressure (hypotension), as well as reduced oxygen diffusion from the capillary membrane to target tissues and reduced oxygen utilization at the cellular level. On the other hand, inflammatory conditions such as sepsis lead to accelerated glycolysis resulting in lactate overproduction [27,28]. Statistical analysis confirmed significantly different values of Lactate in the analyzed time period in preterm newborns with proven sepsis p<0.001. Statistical analysis confirmed significantly different values of PCT in the analyzed time period in newborns with proven sepsis with asphyxia p<0.001 (Figure 4). The highest average values (41.37 ± 62.50) were measured at newborns with proven sepsis with asphyxia during admission with a subsequent sharp jump compared with newborns with proven sepsis with RDS and other comorbidities. Because lactate is a normal product of glucose metabolism, lactate levels can be elevated in many other conditions and in the absence of infection and hypoperfusion, such as malignancy, mitochondrial disorder, diabetic ketoacidosis, trauma, medications and liver disease.
Conclusion
PCT and lactate have prognostic value as biomarkers in patients with sepsis. Together, these two biomarkers provide early detection, information on the severity of sepsis, efficacy in the use of antibiotic therapy, and assessment of outcome in critically ill patients with sepsis.
33226
References
- Kliegman RM, Stanton B, Geme JS, st Schor NF, Behrman RE (2015) Nelson textbook of paediatrics (20th edn). Philadelphia (PA): Elsevier Health Sciences.
- Whicher J, Bienvenu J, Monneret G (2001) Procalcitonin as an acute phase marker. Ann Clin Biochem 38: 483-493.
- Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181: 176-180.
- Müller B, White JC, Nylén ES, Snider RH, Becker KL, et al. (2001) Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 86: 396-404.
- Chiesa C, Panero A, Rossi N, Stegagno M, de Giusti M, et al. (1998) Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis 26: 664-672.
- Christ-Crain M, Müller B (2005) Procalcitonin in bacterial infections: hype, hope, more or less? Swiss Med Wkly 135: 451-460.
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY (2006) Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 34: 1996-2003.
- Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL (1998) Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet 351: 1211-1212.
- Carrol ED, Thomson AP, Hart CA (2002) Procalcitonin as a marker of sepsis. Int J Antimicrob Agents 20: 1-9.
- Floriańczyk B (2003) Structure and diagnostic value of procalcitonin. Ann Univ Mariae Curie Sklodowska Med 58: 338-342.
- Maruna P, Nedelnikova K, Gurlich R (2000) Physiology and genetics of procalcitonin. Physiological Research 49: S57-62.
- Carrol ED, Thomson AP, Hart CA (2002) Procalcitonin as a marker of sepsis. Int J Antimicrobe Agents 20: 1-9.
- Long B, Koyfman A (2017) Ready for prime time? Biomarkers in sepsis. Emerg Med Clin North Am 35: 109-122.
- Sager R, Kutz A, Mueller B, Schuetz P (2017) Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC medicine 15: 15.
- Balci C, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B (2003) Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care 7: 85-90.
- Vincent JL, e Silva AQ, Couto L, Taccone FS (2016) The value of blood lactate kinetics in critically ill patients: a systematic review. Critical care 20: 257.
- Snow T, Maher C, Feiner B, Baessler K, Bai SW, et al. (2012) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Scottish Medical Journal 59: e22-36.
- Freund Y, Delerme S, Goulet H, Bernard M, Riou B, et al. (2012) Serum lactate and procalcitonin measurements in emergency room for the diagnosis and risk-stratification of patients with suspected infection. Biomarkers 17: 590-596.
- Young LS (2005) Sepsis syndrome. In: Mandell GL, Bennett JE, Dolin R (editors). Principles and Practice of Infectious Diseases. Philadelphia: Churchill livingstone.
- Remington JS, Klein JO (2001) Bacterial sepsis and meningitis. Infectious diseases of the fetus and newborn infant. Philadelphia: W.B. Saunders Company.
- Kallel S, Abid M, Jarraya A, Abdenadher M, Mnif E, et al. (2012) Kinetics, diagnostic and prognostic value of procalcitonin after cardiac surgery. Ann Biol Clin (Paris) 70: 567-580.
- Levy B (2006) Lactate and shock state: the metabolic view. Curr Opin Crit Care 12: 315-321.
- Vincent JL (2000) Procalcitonin: The marker of sepsis? Crit Care Med 28: 1226–1228.
- Athhan F, Akagunduz B, Genel F, Bak M, Can D (2002) Procalcitonin: A marker of neonatal sepsis. J Trop Pediatr 48: 10-14.
- Ugarte H, Silva E, Mercan D, de Mendonça A, Vincent JL (1999) Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 27: 498-504.
- Hansson LO, Lindquist L (1997) C-reactive protein: its role in the diagnosis and follow-up of infectious diseases. Curr Opin Infect Dis 10: 196-201.
- Yunus, Iram, Fasih A, Wang Y (2018) The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PloS one 13: e0206527.
- Jan B, de Backer D, Hernandez G (2016) Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med 42: 472-474.











