Keywords
Stroke, coagulopathy, D-dimer, C-reactive protein, coronavirus, Covid-19.
Introduction
The disease caused by the novel coronavirus (2019-nCoV or SARS-Cov-2) was named by the acronym COVID-19 which means “COrona VIrus Disease”, while “19” refers to the year 2019, when the first cases in Wuhan, China, were identified. In the last two decades, two coronavirus epidemics have occurred, in China and Saudi Arabia, the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), whose mortality was, respectively, 10% and 37% [1].
Patients infected with SARS-Cov-2 have symptoms of respiratory infection, such as dry cough, fever, odynophagia, dyspnea. Thrombotic events may be the initial symptom of COVID-19, but other neurological symptoms need to be investigated. Therefore, it is necessary to know about viral pathophysiology, which is still limited [2]. Transmission of 2019- nCoV from humans to humans has been confirmed in China and the USA and occurs mainly with the contact of respiratory droplets from infected patients [3].
The neuroinvasive propensity of SARS-CoV-2 seems to be explained by its genomic similarity with SARS-CoV and MERSCoV, which are known to have access to the central nervous system through the olfactory nerves and subsequently reach specific areas of the brain, including thalamus and brain stem, inducing neurological symptoms [4,5]. Hematogenous dissemination to the cerebral circulation or cribriform plaque and olfactory bulb are other explanations for the penetration of SARS-CoV-2 into the central nervous system (CNS) [6].
Among the neurological changes of Covid-19 is ischemic stroke. There are some hypotheses for its occurrence: (a) invasion of the vascular wall through the angiotensin-converting enzyme receptor 2 (ACE2), inflaming and even necrotizing the wall of the cerebral arteries; (b) general inflammatory state or "cytokine storm" produced by the virus in some patients in stages II and III of the disease, determining its pro-coagulant effect; (c) myocardial damage that culminates in brain cardioembolic changes; and (d) associated systemic inflammation that may destabilize an atheromatous plaque and rupture the fibrous capsule exposing thrombogenic material [7]. Only one study investigated the presence of the virus in CSF [5].
As new cases are reported, more knowledge is gained about SARS-Cov-2. Although there is much research on COVID-19, further studies are needed on the neurological repercussions of this infection, in view of the proven neuroinvasive power of the virus. Thus, the objective of this systematic review was to analyze laboratory changes and management of patients with COVID-19 and stroke.
Patients and Methods
This was a systematic review of the literature developed according to the PRISMA recommendation [8]. We sought to answer the research question: “What are the main hematological changes and therapeutic management of patients with COVID-19 and ischemic stroke?
Based on literature search in the medical databases PubMed, LiLacs, WoS and IBECS, in addition to secondary searches and gray databases of the literature (Google Scholar and OpenGrey), we analyzed in the period from March to May 2020 all case reports on COVID-19 and ischemic stroke, regardless of publication time.
Search descriptors [“cerebral infarction” OR “stroke” OR “brain ischemia” OR “thrombosis” AND “coronavirus; “SARSCov- 2”, “Covid-19”] were in accordance with the PICOS strategy (Patient, Intervention, Comparison, Outcomes and Study) [9]. The population comprised adults of both sexes who tested positive for COVID-19. The primary outcomes were hematological changes and therapeutic management.
Complete original primary studies were included, available online in the selected databases and published in any language, regardless of publication time. Literature reviews, editorials or studies in which it was not possible to identify a relationship with the theme and duplicates in the databases were excluded.
Search was carried out in pairs, with four authors (BLFB, SNMANB, AGM and CHCLC), who independently selected the titles of the articles and then proceeded to read the abstracts and texts in full. A form developed by the authors was used to extract the following data: authors, year, method, population and sample, age, sex, risk factors for ischemic stroke, days from Covid-19 symptoms to stroke, laboratory changes, treatments and clinical outcomes.
A tool was used to assess the quality of case studies, consisting of four domains: selection, verification, causality and communication [10]. According to the recommendation, a general judgment was made on the methodological quality and it was confirmed that the 12 case studies of this selection showed satisfactory quality. The Newcastle-Ottawa Scale was used for observational studies, which consists of three domains: selection, comparison and results; and three evaluation scores: strong evidence (6 to 9 points), moderate evidence (4 to 5 points) and limited evidence (<4 points) [11]. The observational study by Yaghi et al. [12] received a score of 8, which is considered to be strong evidence
Data are presented as arithmetic mean with standard derivation or as percentages. Percentage is always related to the total number of patients on whom information was available for the specific issue. All collected data were organized in database. The BioEstat version 5.0 for statistical analysis was used.
Results
One hundred and seventy-four articles were identified, but after eliminating duplicate articles in the databases or that fit the exclusion criteria, only 12 studies were selected for analysis, according to the flowchart (Figure).
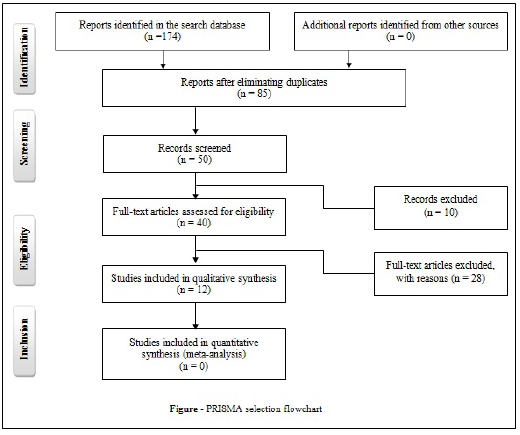
Figure : PRISMA selection flowchart.
In 12 articles analyzed in this review, a total of 52 patients (67.3% men and 32.7% women) with Covid-19 had ischemic stroke with an average age of 57.49 ± 14.4 years, ranging between 31 and 87 years. Neurological symptoms appeared 10.7 ± 8.1 days after the diagnosis of Covid-19, as shown in Table 1.
| Variables |
Number of patients |
| Sex |
|
| Male (n;%) |
35 (67.3) |
| Female (n;%) |
17 (32.7) |
| Age (years) |
|
| Mean (SD) |
57.4 (14.4) |
| Variation |
31-87 |
| Days from Covid-19 symptoms to stroke (mean; SD) |
10.7 (8.1) |
Table 1: Distribution of sex, age and number of days from Covid-19 symptoms to stroke in 52 patients with ischemic stroke.
The main laboratory changes were C-reactive protein (80.8%; 42/52), D-dimer (78.9%; 41/52), and ferritin (13.5%; 7/52).
The therapeutic measures used were anticoagulation (65.4%), anti-platelet aggregation (19.2%), thrombectomy (15.4%), thrombolysis (9.6%), and antithrombotics (7.7%) (Table 2).
| Published studies |
Laboratory findings |
Treatment |
Outcome |
| D-dimer (ug/mL) |
CRP (mg/L) |
PT (sec) |
aPTT (sec) |
INR |
Platelet count |
Serum ferritin |
| Saiegh et al., 2020 [5] |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
thrombectomy |
Rehabilitation |
| Zhou et al., 2020 [13] |
0.83 |
42.5 |
Nl |
Nl |
NR |
↓ |
NR |
anti-platelet aggregation |
Rehabilitation |
| Morassi et al., 2020 [14] |
7.74 |
175.0 |
NR |
↑ |
1.62 |
↓ |
NR |
anti-platelet aggregation |
death |
| NR |
46.0 |
NR |
NR |
1.53 |
NR |
NR |
only mechanically ventilated |
death |
| NR |
181.0 |
Nl |
Nl |
Nl |
Nl |
NR |
anti-platelet aggregation, anticoagulation |
death |
| 1.38 |
12.0 |
NR |
NR |
NR |
NR |
NR |
anti-platelet aggregation, anticoagulation |
Rehabilitation |
| Deliwala et al., 2020 [15] |
36.4 |
161.5 |
NR |
NR |
NR |
Nl |
437.0 |
anti-platelet aggregation, anticoagulation |
Rehabilitation |
| Hughes, 2002 [16] |
NR |
20.0 |
11.2 |
19.7 |
NR |
Nl |
NR |
anticoagulation |
Rehabilitation |
| Tunç et al., 2020 [17] |
0.80 |
142.0 |
NR |
NR |
NR |
NR |
264.0 |
antithrombotics |
Rehabilitation |
| 1.04 |
4.0 |
NR |
NR |
NR |
NR |
79.0 |
antithrombotics |
Rehabilitation |
| 0.64 |
33.0 |
NR |
NR |
NR |
NR |
132.0 |
antithrombotics |
Rehabilitation |
| 0.38 |
3.7 |
NR |
NR |
NR |
NR |
127.0 |
antithrombotics |
Rehabilitation |
| Goldberg et al., 2020 [18] |
↑ |
NR |
↑ |
NR |
NR |
NR |
↑ |
only mechanically ventilated |
death |
| Valderrama et al., 2020 [19] |
>10.0 |
11.0 |
NR |
NR |
NR |
NR |
588.0 |
thrombolysis, thrombectomy |
Rehabilitation |
| Moshayedi et al., 2020 [20] |
NR |
NR |
NR |
>85.5 |
NR |
Nl |
NR |
anticoagulation |
Rehabilitation |
| Viguier et al., 2020 [21] |
2.22 |
219.0 |
NR |
NR |
NR |
Nl |
1,096.0 |
anticoagulation |
Rehabilitation |
| Barrios-López et al., 2020 [22] |
105.0 |
NR |
13.6 |
23.5 |
NR |
Nl |
1,323.5 |
NR |
death |
| 7.59 |
NR |
11.8 |
24.5 |
NR |
Nl |
1,298.7 |
anticoagulation |
Rehabilitation |
| 0.51 |
NR |
11.6 |
24.3 |
NR |
Nl |
65.4 |
NR |
death |
| 0.72 |
NR |
11.7 |
31.7 |
NR |
Nl |
202.8 |
anticoagulation |
Rehabilitation |
Table 2: Distribution of laboratory findings of 52 patients with coronavirus disease 2019 (Covid-19) and ischemic stroke with their respective treatments and outcome.
Risk factors for ischemic stroke were present in 76.9% of patients. The most frequent were: arterial hypertension (76.9%), diabetes mellitus (36.5%), hyperlipidemia (32.7%), and cardiopathy (30.8%). Most patients had two or more risk factors (65%), as shown in Table 3.
| Risk factors |
| Presence (%) |
|
| Yes |
76.9 |
| No |
23.1 |
| Frequency (%) |
|
| Arterial hypertension |
57.7 |
| Diabetes mellitus |
36.5 |
| Hyperlipidemia |
32.7 |
| Cardiopathy |
30.8 |
| Prior stroke |
5.8 |
| Smoker |
3.9 |
| Obesity |
1.9 |
| Number of risk factors per patient (%) |
|
| One |
35.0 |
| Two |
27.5 |
| ≥Three |
37.5 |
Table 3: Distribution of risk factors for ischemic stroke in patients with covid and ischemic stroke.
In Table 4, we compared patients with and without risk factors for stroke and found that there was a higher mortality in those with risk factors. This difference was not statistically significant (p=0.33).
| Clinical outcome |
Risk factors for ischemic stroke |
p-value |
| Yes (n=40) |
No (n=12) |
| Discharged to rehabilitation (n; %) |
17 (73.9) |
6 (26.1) |
0.33 |
| Critically ill (n; %) |
6 (66.7) |
3 (33.3) |
| Death (n; %) |
17 (85.0) |
3 (15.0) |
Table 4: Distribution of clinical outcomes according to risk factors for ischemic stroke.
p-value calculated using the Fisher's exact test, comparing: discharged to rehabilitation and critically ill versus death
Discussion
This review showed the demographic and laboratory characteristics of patients with COVID-19 who evolved with stroke. The results were analyzed with caution, as there were limitations in the studies. First, most articles were case studies, which have low power of generalization, despite good methodological quality. Second, the number of patients analyzed was very small. Third, most studies did not provide complete information about laboratory findings or imaging studies. Finally, studies were limited to cerebral ischemia. COVID-19 has become a pandemic that defies public health, due to its rapid spread and its various complicating outcomes related to organic systems, including the CNS. Infection of the CNS increases the probability of the patient to have a stroke by 1.4 times, particularly at the beginning of convalescence. In SARS-CoV-2 infection, a similar probability is also expected [19]. There is a description that 6% of severely affected patients may develop acute cerebrovascular disease [23]. This discussion will be divided into three topics: characterization of patients with COVID-19 and AVCI, main laboratory findings, and clinical management of these patients.
Characterization of patients with COVID-19 and stroke
Twelve studies involving 52 patients with COVID-19 who had stroke were analyzed. The results showed that men were twice more affected than women and that age does not seem to have influenced the occurrence of stroke, since those over 60 years of age are more affected, especially when they have prothrombotic risk factors [17]. COVID-19 is known to induce hypercoagulable states by disturbing hemostasis pathways. Viral load seems to be the contributing factor for coagulopathy and endothelial dysfunction [15]. Risk factors for stroke, which predominated in this study, are proven to be complicating variables in the clinical outcomes of SARS-Cov-2 infection. Hypertension, diabetes mellitus, hyperlipidemia and heart disease occurred more frequently. There is evidence that cardiovascular risk factors stimulate an increase in inflammatory response biomarkers, as well as a hypercoagulable state. In addition, in the presence of systemic inflammation and sepsis, there is possibly an increased vulnerability of atherosclerotic plaques to rupture, contributing to complications in the acute phase of COVID-19 [14].
Neurological symptoms occurred, on average, 10.7 days after the diagnosis of COVID-19, coinciding with the inflammatory phase of the disease, which is characterized by a storm of proinflammatory and pro-coagulant cytokines. This storm of cytokines with platelet activation, endothelial dysfunction and blood stasis predisposes to thrombosis in the venous and arterial circulations and may progress to disseminated intravascular coagulopathy and thrombotic microangiopathy. This pattern of presentation of coagulapathy seems to be responsible for the AVCI episodes in COVID-19 [5].
Main laboratory findings
An overview of the main laboratory changes in these patients was presented in Table 2. An increase in D-dimer was observed in 78.9% (41/52) of the patients [12-15,17-19,21,23]. D-dimer is an indirect marker of thrombin synthesis and increases in coagulopathies associated with inflammatory conditions. The measurement of your serum level can help in the early recognition of high-risk patients, since high serum levels (three or four times the normal value of 0.5μg / mL) on hospital admission may mean progression to phases II and III of COVID-19, even in the absence of other serious symptoms [24].
A study showed that D-dimers in series increased in the days leading to the stroke, indicating a state of hypercoagulation [15]. Based on this, it is recommended that all patients with COVID-19, seriously ill or with high levels of D-dimer, be treated with thromboprophylaxis [25]. It was shown that in patients with COVID-19 who had high levels of D-dimer and used anticoagulants, there was a reduction in cases of stroke [12].
On the other hand, it was suggested that D-dimer might not be able to reflect the exact fibrinolysis status of patients with COVID-19 and, therefore, could not guide the possible antifibrinolysis or thrombolytic therapy at different stages of the disease. It is necessary to investigate whether the measurement of direct markers of thrombin, plasmin, among others, could provide more therapeutic targets in patients with COVID-19 and coagulopathy [26].
There was an increase in C-reactive protein (CRP) in 80.8% (42/52) of the patients [12,17,19,21]. It is a marker of the inflammatory process that may cause the amplification of the immune response, leading to increased tissue damage. Increased production of CRP by the liver occurs in acute infectious processes or in non-infectious conditions, such as ischemic stroke. During an inflammatory response in atherosclerosis, the involvement of CRP in the process of endothelial dysfunction is detected, by stimulating the production of adhesion molecules and chemokines in the endothelium [24].
In most studies, the evaluation of the coagulogram was not reported, making it difficult to infer the relationship between these factors and the presence of cerebral coagulopathy. An increase in prothrombin time, activated partial thromboplastin time and international normalized ratio (INR) and thrombocytopenia was observed in, respectively, 11.5% [16,18,22], 3.9% [14,20], 3.9% [14], and 3.9% [13,14] of patients. In the presence of a patient with thrombocytopenia, prothrombin time should be evaluated, which is an important marker of disease severity. The International Society for Thrombosis and Hemostasis recommends that serum fibrinogen be measured to assess the possibility of disseminated intravascular coagulopathy [24].
Severe cases of COVID-19 showed high levels of D-dimer and thrombocytopenia, making these patients prone to stroke [5]. This pro-thrombotic characteristic of COVID-19 is due to the viral involvement of the integrity of the endothelium and the balance between coagulation and anticoagulation, and to provoke an inflammatory response. Tudo isso culminará em hipercoagulação e trombose [13].
Ferritin is a marker of inflammatory response and is elevated in antiphospholipid syndrome and its variant, which is associated with coagulopathy and arterial and venous thrombosis. In this review, increased serum ferritin levels were found in 13.3% (7/52) of patients [15,17-19,21,22]. The increase in serum ferritin increases the chances of mortality in patients with COVID-19 [18], but this was not confirmed in our study.
Clinical management of patients with COVID-19 and stroke
The main clinical manifestations of COVID-19 are respiratory, but cardiovascular, thrombotic and ischemic complications resulting from inflammatory processes and hypercoagulable state frequently occur. Therefore, there are several consensus suggesting prophylactic treatment with low molecular weight heparin during the admission of these patients and for 7 to 14 days after hospital discharge [27-30].
According to the International Society for Thrombosis and Hemostasis, the prophylactic use of low molecular weight heparin should be performed in all patients who are admitted with COVID-19, except those who have a contraindication (active bleeding and platelet count below 25x109/L) [31]. On the other hand, these patients may show resistance to heparin, in an acute phase response, developing a prohemostatic effect that may antagonize the anticoagulant effects of heparin [32].
Anticoagulation, both therapeutic and prophylactic, was administered to most patients [12,14,15,20-22]. Good therapeutic response was found in some patients, through clinical improvement and confirmed by the reduction of the National Institute of Health Stroke Scale (NIHSS) [12,16,21].
In this review, anticoagulation (65.4%), anti-platelet aggregation (19.2%), thrombectomy (15.4%), thrombolysis (9.6%), and antithrombotics (7.7%) were therapeutic measures used. The high mortality rate (20/52; 38.5%) seems not to have been influenced by the therapeutic choice, but by the presence of risk factors (85% versus 15%). On the other hand, many stroke patients did not have the opportunity to be treated with thrombolysis or thrombectomy, because they are very ill [14]. Early treatment, up to four hours after the event, prevents extensive tissue ischemia, reducing adjacent neurological damage.
Conclusions
COVID-19 patients have laboratory alterations compatible with an inflammatory response and a state of hypercoagulability, considered a potential cause of ischemic stroke.
39898
References
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status Mil Med Res 7: 11.
- Wang D, Hu B, Hu C, Fangfang Z, Xing L (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan China JAMA 323: 1061-1069.
- Singhal T (2020) A Review of Coronavirus Disease-2019 (COVID-19) Indian J Pediatr 87: 281-286.
- Li Y-C, Bai W-Z, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients J Med Virol 92: 552-555
- Saiegh FA, Ghosh R, Leibold A, Avery MB, Schmidt RF (2002) Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke J Neurol Neurosurg Psychiatry 91: 846-848.
- Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K (2020) Neurological manifestations of COVID-19 and other coronavirus infections A systematic review Clin Neurol Neurosurg 194: 105921.
- Trejo-Gabriel-Galán JM (2002) Stroke as a complication and prognostic factor of COVID-19 Neurología 35: 318-322.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses The PRISMA statement PLoS Med 6: 1000097.
- Eriksen MB, Frandsen TF (2018) The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality a systematic review. J Med Libr Assoc 106: 420-431.
- Murad MH, Sultan S, Haffar S, Bazerbachi F (2018) Methodological quality and synthesis of case seriesand case reports BMJ Evid Based Med 23: 60-63.
- Wells G, Shea B, O'Connell DL, Peterson J, Welch V The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Yaghi S, Ishida K, Torres J, Grory BM, Raz E (2020) SARS-CoV-2 and stroke in a New York Healthcare System Stroke 51: 2002-2011.
- Zhou B, She J, Wang Y, Ma X (2002) A case of coronavirus disease 2019 with concomitant acute cerebral infarction and deep vein thrombosis Front Neurol 11: 296.
- Morassi M, Bagatto D, Cobelli M, D–Agostini S, Gigli GL (2020) Stroke in patients with SARS-CoV-2 infection: Case series J Neurol 267: 2185-2192.
- Deliwala S, Abdulhamid S, Abusalih MF, Al-Qasmi MM, Bachuwa G (2020) Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (COVID-19) Cureus 12: 8121.
- Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S (2020) Cerebral venous sinus thrombosis as a presentation of COVID-19 Eur J Case Rep Intern Med 7: 001691.
- Tunç A, Ünlübaş Y, Alemdar M, Akyüz E (2020) Coexistence of covid-19 and acute ischemic stroke report of four cases J Clin Neurosci 77: 227-229.
- Goldberg MF, Goldberg MF, Cerejo R, Tayal AH (2020) Cerebrovascular disease in COVID-19 Am J Neuroradiol. doi:10.3174/ajnr.A6588.
- Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S (2020) Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke Stroke 51: 124-127.
- Moshayedi P, Ryan TE, Mejia LLP, Nour M, Liebeskind DS (2020) Triage of acute ischemic stroke in confirmed COVID-19 Large vessel occlusion associated with coronavirus infection Front Neurol 11: 353.
- Viguier A, Delamarre L, Duplantier J, Olivot JM, Bonneville F (2020) Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection J Neuroradiol 47: 393-394
- Barrios-López JM, Rego-García I, Muñoz Martínez C, Romero-Fábrega JC, Rivero Rodríguez M (2020) Ischemic stroke and SARS-CoV-2 infection A causal or incidental association? Neurologíaa 35: 295-302.
- Mao L, Jin H, Wang M, Hu Y, Chen S (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan China JAMA Neurol 77: 1-9.
- Brandão SCS, Tenório E, Silva AGBB, Ramos JOX, Melo LMMP (2020) Covid-19, imunidade, endotélio e coagulação: compreenda a interação Accessed on: July 26, 2020.
- Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS (2020) Thromboembolic risk and anticoagulant therapy in COVID-19 patients emerging evidence and call for action Br J Haematol 189: 846-847.
- Tang N, Bai H, Xiong D, Sun Z (2020) Specific coagulation markers may provide more therapeutic targets in COVID-19 patients receiving prophylactic anticoagulant J Thromb Haemost 18: 2428-2430.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up J Am Coll Cardiol 75: 2950-2973.
- Marietta M, Ageno W, Artoni A, De Candia E, Gresele P COVID-19 and haemostasis a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus 18: 167-169.
- Song JC, Wang G, Zhang W, Zhang Y, Li WQ (2020) Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19 Mil Med Res 7: 19.
- Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z (2020) Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines Thromb Haemost 120: 937-948.
- Thachil J, Tang N, Gando S, Falanga A, Cattaneo M (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19 J Thromb Haemost 18: 1023–1026.
- Thachil J, Juffermans NP, Ranucci M, Connors JM, Warkentin TE (2020) ISTH DIC Subcommittee Communication on Anticoagulation in COVID-19 J Thromb Haemost 18: 2138-2144.






