Keywords
Susceptibility pattern, Resistance pattern, Multi drug resistant microorganisms, Sources for wound contamination, ATCC standard strains
Introduction
Contamination is the attack of the body by infection bringing about pathogen that get to be set up, increase in the body and in this way deliver side effects [1]. On the off chance that such pathogens defile and colonize the injury then twisted contamination is brought on. Wound contamination is characterized as the arrangement of discharge in an injury, and in addition other general or neighborhood components of sepsis including pyrexia, torment and in terms [2]. Wound diseases represent 70-80% death rate [3]. Wound may be countered in clinical practice either postoperatively, taking after injury, or could principally be of infective birthplace [4]. Despite their starting point, all injuries may debased by microorganisms or outside bodies or both [5]. Wound tainting sources incorporate : (i) nature (exogenous microorganisms noticeable all around or those presented by traumatic damage), (ii) the encompassing skin (including individuals from the ordinary skin microflora, for example, Staphylococcus epidermidis, micrococci, skin diphtheroids, and propionibacteria), and (iii) endogenous sources including mucous layers (fundamentally the gastrointestinal, oropharyngeal, and genitourinary mucosae)[6]. The part and essentialness of microorganisms in wound mending has been bantered for a long time, a few specialists consider the microbial thickness to be basic in anticipating wound recuperating and disease While others consider the sorts of microorganisms to be of more prominent hugeness. Albeit wound contaminations are brought on by microorganisms, broad debate still exists with respect to the system by which they cause disease. The greater part of open injury colonization is polymicrobial [7-9], including various microorganisms that are potentially pathogenic. However twisted consideration experts considered that high-impact or facultative pathogens, for example, Staphylococcus aureus, Pseudomonas aeruginosa, and beta-hemolytic streptococci and discharge framing pathogens like Enterococci sp, Escherichia coli, Klebsiella sp. also, Proteus sp [10] are the essential drivers of postponed recuperating and disease in both intense and constant injuries. As indicated by a writing anaerobic microorganisms include, 33% of the aggregate number of microbial species in colonized injuries, and their number increments to around half in contaminated injuries. Consequently, antimicrobial treatment of clinically tainted injuries ought not just target particular pathogens that are normally thought to be the causative specialists (e.g., S. aureus and P. aeruginosa) however ought to cover an assortment of conceivably synergistic oxygen consuming or facultative and anaerobic microorganisms. For instance alone metronidazole or clindamycin cover just the anaerobic parts, in this manner show Poor achievement rates [11]. Blend treatment with an aminoglycoside (e.g., gentamicin) or a cephalosporin (e.g., cefuroxime or cefotaxime) in addition to clindamycin or metronidazole has turned out to be extremely compelling. Since S. aureus is thought to be the most widely recognized pathogen included in contaminated injuries [12,13] cephalosporins, macrolides, clindamycin, and semisynthetic penicillin's, for example, flucloxacillin and oxacillin are generally medicines of decision [12]. On the off chance that strains of MRSA are included, the glycopeptide anti-toxins vancomycin and teicoplanin are elective decisions. Henceforth the treatment of contamination is required to pick the right sort of anti-toxins and the fitting fixations to be utilized, thinking seriously about the etiology of the disease and the span of the anti-microbial treatment to avert anti-toxin resistance [14].
The target of this paper is to substantiate the bacterial pathogens disengaged from discharge and wound specimens. In light of the way that Selection of a successful antimicrobial operators for a microbial contamination requires information of the potential microbial pathogen, accordingly fitting anti-toxins, the enchantment shots or the phenomenal medications can be utilized to recuperate the injuries and in this manner the intricacies, which are a danger to all patients and hence can be minimized as it were. Furthermore the assessment of particular microorganisms disengaged from an injury may be translated by the professional as a determination of wound disease that requires suitable antimicrobial treatment which will help in decreasing the microbial resistance.
Bacterial strains
Fundamentally Study composed on review information of recent years from Intensive Care Units of open and private Health Care Sector of Critically Ill patients. For that reason Sensitivity and Resistance example of most pervasive Microorganisms, more than 200 confines were gotten from Antibiograms of healing facilities. Disengages got were for the most part doctor's facility obtained with little group gained. Copy segregate meet the rejection criteria and Non Duplicate disconnects antibiogram were translated on results.
Organism identification
All pathogens were identified at the participating center using routine methods for that laboratory and were confirmed at the coordinating Laboratory.
Susceptibility testing
Methods of Sensitivity testing performed by participating hospital was based on: Disk Diffusion Method of Kirby-Bauer; VITEK (biomerieux vitek, Hazlwood MO); with micro broth dilution of cation adjusted Muller-Hinton broth (CAMHB) and colonies suspended on them were equivalent to a 0.5 McFarland Standard. The resulting antibiograms interpretated according to CLSI reference standard.
Antimicrobial agents
Antimicrobials sensitive to pathogens were interpret on final result like Quinolones, Cephalosporin, Amino glycosides, and Beta-lactamase inhibitors, Carbapenems, Monobactams, Antiinfective, Macrolides, and Colisthemetate.
Zone size inhibition measurement for interpretation of Susceptibility and Resistance pattern were based on standard content disc of Piperacillin/tazobactam 100/10 μg, Parenteral Cephalosporins 30 μg (ceftazidime, cefepime), Aztreonam 30 μg, Carbapenems 10 μg, Colisthemetate 10 μg, Amikacin 30 μg, Gentamycin and Tobramycin 10 μg with Ciprofloxacin 5 μg respectively with reference standard according to CLSI of zone of sensitivity and resistance measurement.
Quality control
Resulting outcomes were compared with ATCC standard strains.
Result and Statistical Analysis
Approximately 200 isolates of different pathogens are collected to determine the sensitivity and resistance pattern of different pathogens causing wounds, sepsis and other infections in intensive care unit. various samples are collected for isolation of certain pathogens includes: pus c/s, pus swab, wound c/s were among the major source of isolation of these infectious pathogens in intensive care units shown in Graph 1 and Table 1.
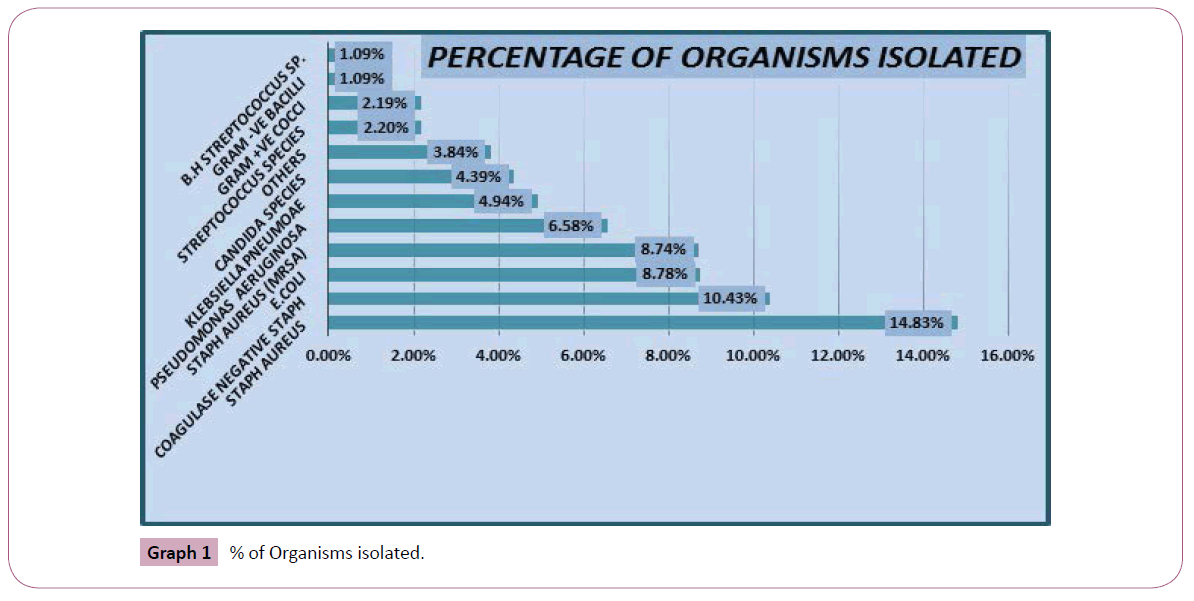
Graph 1: % of Organisms isolated.
| Examples of potential wound pathogens |
| Gram-positive cocci |
•Beta Haemolytic Streptococci (Streptococcus pyogenes)*
•Enterococci (Enterococcus faecalis)
•Staphylococci (Staphylococcus aureus/MRSA)* |
| Gram-negative aerobic rods |
•Pseudomonas aeruginosa* |
| Gram-negative facultative rods |
•Enterobacterspecies
•Escherichia coli
•Klebsiellaspecies
•Proteus species |
| Anaerobes |
•Bacteroides
•Clostridium |
| Fungi |
•Yeasts (Candida)
•Aspergillus |
Table 1: Examples of wound pathogens.
After the data collection from intensive care units, the susceptibility and resistance pattern of antimicrobials were analyzed as per respect to their increasing frequency day by day due to which antibiotic emergence and the infrequent use of antibiotics against pathogens developed.
Our result had showed that due to increasing frequency in consumption of antimicrobials, the resistance progression and the trend to high class of antimicrobials is increased. Table 2 clearly illustrate the increasing frequency of resistance and sensitivity of antibiotics for pathogens.
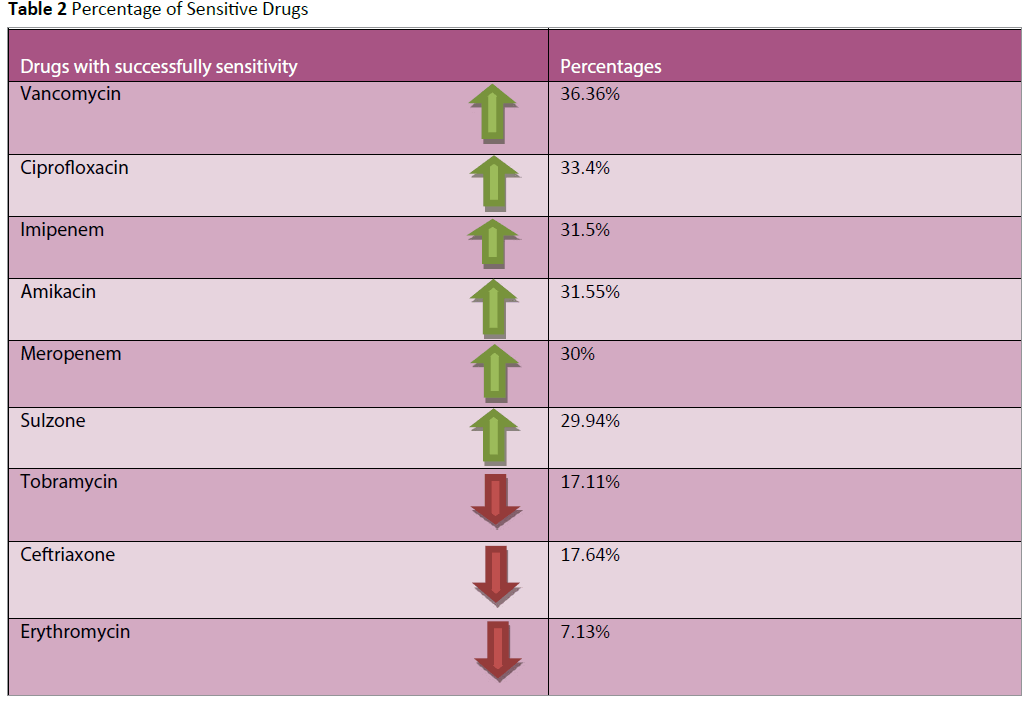
Table 2: Percentage of Sensitive Drugs
The sensitivity pattern for pathogens is quite higher with vancomycin 36.3%, ciprofloxacin 33.40%, imipenem 31.55%, meropenem 30% (Table 2). Graph 3 showed the Percent frequency of antimicrobial resistance for isolated pathogens was ceftriaxone 29.41%, ciprofloxacin 26.09% erythromycin 26.30%, tobramycin 25.66% respectively. Beta Haemolytic Streptococci (Streptococcus pyogenes), Enterococci (Enterococcus faecalis), Staphylococci (Staphylococcus aureus/MRSA) were the most potential wounds of gram positive cocci (Table 1).
Graph 1 revealed that staph aureus and coagulase negative staph were the organisms which were abundantly present with 14.83% and 10.43% respectively. The comparative result revealed that the vancomycin, ciprofloxacin, imipenem, meropenem are the drugs with highest sensitivity pattern whereas erythromycin, ceftriaxone and ciprofloxacin are the drugs considered to be highly resitant against pathogenic wounds (Table 3).
| Drug |
Sensitivity |
Resistance |
| PENICILLINS |
| Cloxacillin |
17.65% |
17.60% |
| Piperacillin/Tazobactum |
24.06% |
20.85% |
| CEPHALOSPORIN |
| Cefixime |
3.20% |
17.11% |
| Ceftriaxone |
17.64% |
29.41% |
| Sulzone |
29.94% |
12.83% |
| Cefipime |
14.97% |
21.39% |
| AMINOGLYCSIDES |
| AmikacinSulphate |
31.55% |
18.71% |
| Gentamycin |
12.83% |
9.09% |
| Tobramycin |
17.11% |
25.66% |
| QUINOLONES |
| Ciprofloxacin |
33.40% |
26.09% |
| Pipemidic acid |
1.09% |
12.00% |
| MACROLIDES |
| Erthromycin |
7.13% |
26.30% |
| MONOBACTUM |
| Azetreonam |
10.01% |
10.69% |
| CARBAPENEM |
| Imipenem |
31.51% |
11.23% |
| Meropenem |
30% |
12.29% |
| GLYCOPEPTIDE |
| Vancomycin |
36.36% |
0% |
| Teicoplanin |
13.90% |
16.04% |
Table 3: Percentage of antimicrobial resisitance and sensitivity against pathogens.
Graph 2 revealed that vancomycin is highly sensitive among all antibiotics against pathogens and then ciprofloxacin, imipenem respectively. Whereas Graph 3 showed that ceftriaxone is highly resistant towards pathogens and then ciprofloxacin, tobramycin, erythromycin respectively. It has been showed that staph aureus and coagulase negative staph are the organisms which are highly present in sepsis and wounds. Against pathogens the comparative analysis revealed that there is a very close bonding between the utilization of antimicrobials and the resistance and susceptibility pattern in the past years in ICU of hospitals due to which the antimicrobials emergence is created in variety of pathogens.
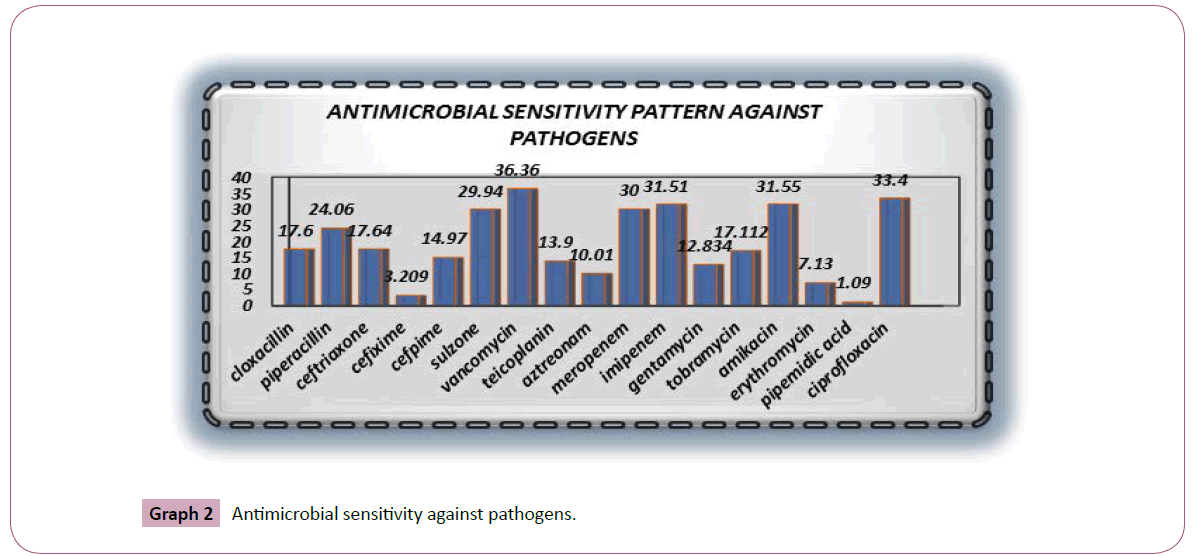
Graph 2: Antimicrobial sensitivity against pathogens.
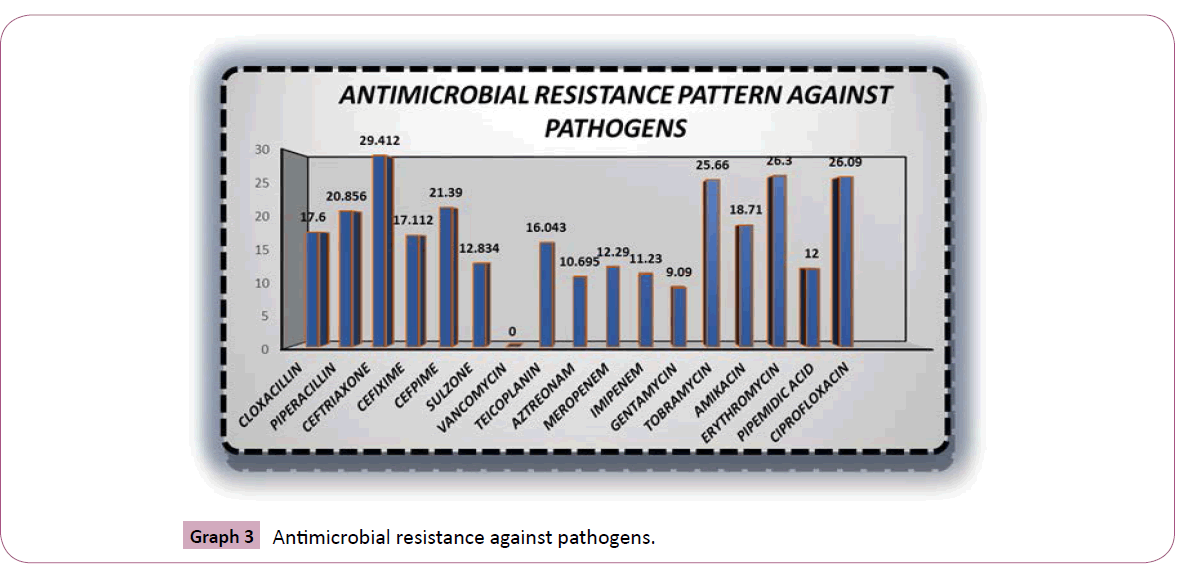
Graph 3: Antimicrobial resistance against pathogens.
Discussion
Despite of the gradual progress in surgery, surgical techniques and antibiotic prophylaxis [15-17] surgical wound infections are the commonest complications and one of the most frequently encountered nosocomial infections (HAI) thus they are supposed to be an important cause of morbidity and mortality worldwide [18,19]. The incidence of these infections estimated by the Center for Disease Control and Prevention (CDC) USA and the UK Nosocomial Infection Surveillance is 15.45% and 11.32% respectively [20]. These infections complicate illness, cause anxiety, increase patient discomfort and can lead to death [21,22]. The development of an infection will be influenced largely by the virulence of the organism and immunological status of the patient. Once a diagnosis of wound infection has been verified and antibiotic sensitivities detected, correct management regimens should be considered, with a high precession given to decreasing the chance of cross infection [23]. Since wound colonization involves not only one type of potential pathogen but numerous types of microbes that can cause wound infections [24-27]. Although, it is a widespread opinion among practitioners that primary cause of delayed healing and infection in wounds, are aerobic or facultative pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and beta-hemolytic streptococci. The incidence of occurrence of S. Aureus is high in wounds therefore it is considered as the most problematic [28-32]. But according to a review of the literature colonized wounds contain one-third of anaerobic bacteria while infected wounds contain 50% of anaerobic bacteria. Therefore, antimicrobial treatment of wounds should have coverage of a variety of potentially synergistic aerobic or facultative and anaerobic microorganisms and should not only target specific pathogens that are often supposed to be the causative agents (such as S. Aureus and P. Aeruginosa). Hence both aerobic and anaerobic pathogens may contribute to infection in poly microbial wounds (frequently via combined interactions), broad-spectrum antibiotics assist the most successful treatment in the management of wound infections. As metronidazole or clindamycin alone, target only the anaerobic components thus they exhibit poor success rate [28]. But clindamycin or metronidazole along with an aminoglycoside (e.g., gentamicin) or a cephalosporin (e.g., cefuroxime or Cefotaxime) has proved to be very effective. For the treatment of established infection,the cephamycin or cefoxitin has been widely used as a single agent in the United States. But the origination of new classes of antibiotics such as the ureidopenicillins, the carbapenems, and the b-lactam/b-lactamase inhibitor combinations has enlarged the choice for both prophylactic and therapeutic treatment [32]. Since S. Aureusis supposed to be the most problematic pathogen involved in infected wounds [32,33], most frequent treatments of choice are cephalosporins, macrolides, clindamycin,and semi synthetic penicillins such as flucloxacillin and oxacillin [34]. If strains of MRSA involved, then the glycopeptide antibiotics vancomycin and teicoplanin are alternative choices [32]. In a previous study poly microbial growth detected from 59.6% of cultures and 61.5% multidrug-resistant organisms isolated. Our results are not very much different from previous studies [35,36]. As our most cultures revealed poly microbial and multidrugresistant organisms, the overall antibiotic susceptibility was highly variable [24,25,32].
The selection of treatment for wound infections requires an understanding of the usual infectious flora, available antimicrobial agents and susceptibility patterns of the infecting organisms as these would be beneficial in the selection of empiric antimicrobial therapy and also on infection control zones in the health institutions. The determination of the microbiologic spectrum and antibiotic susceptibility of isolates in surgical would infections is therefore of increasing criticalness bearing in mind the increasing antibiotic suppression by microorganisms and the high cardinal point of surgical infections caused by these resistant organisms [16,17]. An appreciation of the factors involved in the progression of wound from colonization to infection can help practitioners to interpret clinical findings and microbiological investigations of wounds thus may aid in the development of more effective methods of treating infected and poorly healing wounds. As the controversy related to the use of topical antiseptics persists and the development of bacterial resistance to antibiotics continues, the desire for recognition and establishment of new antimicrobial agents that are safe and broadly effective and have a low tendency to induce resistance becomes increased abruptly. However the microbiology of wounds has been dynamically researched in recent years, there is quiet much to be learned about the microbial mechanisms that induce infection and prevent wound healing [16,17,29]. Consequently, debate respecting microbial involvement in wound healing is likely to continue. In imparting a summarized analysis of wound microbiology, together with current opinion and controversies respecting wound evaluation and treatment, this review has attempted to acquire and approach microbiological aspects that are influential to the advantageous management of microorganisms in wounds [23,32,35].
Conclusion
The study presents a clear understanding to the causative pathogens of wound infections in this hospital and their sensitivity and resistance profiles. It has been concluded that wound infections in this were polymicrobic in nature and, in most cases, associated with S. aureus, coagulase negative staph, E.coli and Pseudomonas aeruginosa. Results also displayed that there is a high rate of antibiotic resistance in all pathogens isolated. Of all the antibiotics tested, vancomycin was shown to be the one most likely to be effective in treating infections as, in contrast to other antimicrobial agents tested in this study, not a single bacterial isolate was found to be resistant to its activity. A continuous inspection should be carried out to monitor the susceptibility of these pathogens and chose appropriate regimens both for prophylaxis and treatment of surgical wound infections. There is a need to create a viable antibiotic policy and draft guidelines to prevent or reduce undirected use of antibiotics, and conserve their effectiveness for better patient management. consistent dialogue between the microbiology department and the surgeons is strongly cautioned in keeping with preventing and controlling surgical wound infections at little cost. This will force rational use of antimicrobial agents and help in curbing the unsafeness of resistance to these agents.
8092
References
- Anathanarayan and pankir (2006) Test book of microbiology (7th edn), by Anathanarayan and pankir.
- Shija JK (1976) The incidence and pattern of sepsis among general surgical in-patients at Muhimbili hospital, Dar es Salaam, 1973. East Afr Med J 53: 153-159.
- Wilson AP, Gibbons C, Raeves BC, Hogson B, Liu M, et al. (2004) Surgical wound infections as a performance indicator: agreement of common definition of wound infections in 4773 Patients BMJ 329: 720–722.
- Sule AM, Thanni LO, Sule AO, Olusanya O (2002) Bacterial Pathogenes Associated with Infected wounds in Ogun State UniversityTeaching Hospital, Sagamu ,Nigeria African J of Clinical and Experiemental Microbiology 3: 13–17.
- Bellchambers J, Harris JM, Cullinan P, Gaya H, Pepper JR (1999) A prospective study of wound infection in coronary artery surgery. Eur J CardiothoracSurg 15: 45-50.
- Duerden BI1 (1994) Virulence factors in anaerobes.Clin Infect Dis 18 Suppl 4: S253-259.
- Bowler PG (1998) The anaerobic and aerobic microbiology of wounds: a review. 10: 170–178.
- Bowler PG, Davies BJ (1999) The microbiology of acute and chronic wounds. 11: 72–79.
- Mousa HA1 (1997) Aerobic, anaerobic and fungal burn wound infections. J Hosp Infect 37: 317-323.
- Wren MW, Baldwin AW, Eldon CP, Sanderson PJ (1977) The anaerobic culture of clinical specimens: a 14-month study. J Med Microbiol 10: 49-61.
- Gorbach SL1 (1994) Antibiotic treatment of anaerobic infections.Clin Infect Dis 18 Suppl 4: S305-310.
- Eron LJ1 (1999) Targeting lurking pathogens in acute traumatic and chronic wounds. J Emerg Med 17: 189-195.
- Periti P, Tonelli F, Mini E (1998) Selecting antibacterial agents for the control of surgical infection: mini-review. J Chemother 10: 83-90.
- Rajalakshmi V, Amsaveni V (2012) Antibiotic Susceptibility of Bacterial Pathogens Isolated from Diabetic Patients, International Journal of Microbiological Research 3: 30-32.
- Bowler PG, Duerden BI, Armstrong DG (2001) Wound microbiology and associated approaches to wound management. ClinMicrobiol Rev 14: 244-269.
- Nichols RL1 (2004) Current Strategies for Prevention of Surgical Site Infections. Curr Infect Dis Rep 6: 426-434.
- Surucuoglu S, Gazi H, Kurutepe S, Ozkutuk N, Ozbakkaloglu B (2005) Bacteriology of surgical wound infections in a tertiary care hospital in Turkey. East Afr Med J 82: 331-336.
- Tesfahunegn Z, Asrat D, Woldeamanuel Y, Estifanos K (2009) Bacteriology of surgical site and catheter related urinary tract infections among patients admitted in Mekele Hospital, Mekele, Tigray, Ethiopia. Ethiopian Medical J 47: 117–122.
- Ashby E, Haddad FS, O'Donnell E, Wilson AP (2010) How will surgical site infection be measured to ensure "high quality care for all"? J Bone Joint Surg Br 92: 1294-1299.
- Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ (2002) The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infection Control and Hospital Epidemiology 3: 183–189.
- (2002) NINSS, Surveillance of Surgical Site Infection in English Hospitals: a national surveillance and quality improvement programme. Public Health Laboratory Service.
- Cooper R, Kingsley A, White R (2003) Wound Infection and Microbiology : Medical Communications (UK) Ltd for Johnson & Johnson Medical.
- Collier M (2001) A ten-point assessment plan for wound management. J CommNurs 16: 22-26.
- Bowler PG (1999) The prevalence and significance of anaerobic bacteria in wounds. M.Phil. thesis.University of Wales College of Medicine, Cardiff United Kingdom, 1999.
- Talan PH, Strong C, McTeague M, Bennion R, Thompson JE, et al. (1995) Bacteriology of skin and soft-tissue infections: comparison of infections in intravenous drug users and individuals with no history of intravenous drug use, Clin Infect Dis 20: S279–S282
- Brook I, Frazier EH (1998) Aerobic and anaerobic microbiology of chronic venous ulcers. Int J Dermatol 37: 426-428.
- Mousa HA1 (1997) Aerobic, anaerobic and fungal burn wound infections. J Hosp Infect 37: 317-323.
- Haneke E (1997) Infections in dermatological surgery,InM.Harahap Diagnosis and treatment of skin infections. Blackwell Science, Oxford, United Kingdom 416-430.
- Mayhall CG (1993) Surgical infections including burns, In R. P. Wenzel Prevention and control of nosocomial infections (2nd edn). The Williams & Wilkins Co. Baltimore 614-664.
- Nichols RL, Smith JW (1994) Anaerobes from a surgical perspective. Clin Infect Dis 18 Suppl 4: S280-286.
- Periti P, Tonelli F, Mini E (1998) Selecting antibacterial agents for the control of surgical infection: mini-review. J Chemother 10: 83-90.
- Eron LJ1 (1999) Targeting lurking pathogens in acute traumatic and chronic wounds. J Emerg Med 17: 189-195.
- Men’shikov DD, Zalogueve GV, Bialik IF (1991) The dynamics ofthe wound microflora in the victims of the earthquake in Armenia ZhMikrobialEpidimiolImmunobial 9:29.
- Otokunefor TV, Datubo-Brown DD (1990) Bacteriology of wound infections in the surgical wards of a teaching hospital. West Afr J Med 9: 285-290.
- Uçkay I, Sax H, Harbarth S, Bernard L, Pittet D (2008) Multi-resistant infections in repatriated patients after natural disasters: lessons learned from the 2004 tsunami for hospital infection control. J Hosp Infect 68: 1-8.










