Key words
|
| |
| E.coli, Chicken antibodies (IgY), ELISA |
| |
Introduction
|
| |
| Mastitis caused by bacteria (E. coli, Klebsiella, Enterobactor) found in the cows surroundings. Unlike contagious forms of mastitis which spread cow-to-cow during milking, coliforms come from environmental sources, such as manure and organic material/bedding (recycled manure, wood shavings, etc). Coliform bacteria can enter the teat canal both during and between milking. Dirty udders, especially when wet, have enormous bacterial populations. High rainfall, hot and humid weather, and moist environments can trigger heavy bacterial growth and increase incidence of coli mastitis. Fever, swollen/warm quarter (usually only 1 quarter affected), abnormal milk, decreased appetite, depression, diarrhea, and standing away from other herd-mates are common clinical signs of Coli mastitis. Infections occur both during lactation and the dry period. Most infections occur during the first 2 weeks following dry-off, 2 weeks before freshening, and early (1st 2 months) lactation, although infections can occur at any time. Infections originating during the dry-period usually don’t become noticeable to the dairy farmer until after freshening. Milking wet udders and/or teats greatly increases risk of mastitis. In addition, the teat end does not fully close for 1-2 h after milking which can increase the chance of infections immediately following milking. Maiti et al. (2003) reported 70.37% incidence of sub clinical mastitis in cows. Mastitis causes heavy economic losses to the dairy industry worldwide. Apart from its economic importance it is also a matter of concern of carries public health significance. Moreover, presence of antibiotic residues in the milk is undesirable due to its public health concern (Sudhan et al., 2010). Peak bacterial numbers have usually already occurred when signs of mastitis appear, so antibiotic therapy is of little to no benefit. Till date broad spectrum antibiotics are injected to reduce financial loss. It leads to serious side effects. |
| |
| Therefore, attention is being paid to find alternative approaches. Recent research showed that chicken egg yolk antibodies will act as promising alternative for diagnosis and treatment of mastitis causing organisms. Zhen et al. (2008) reported that The specific IgY against mastitis-causing Stapylococcus aureus inhibited the growth of Staph. aureus and enhanced the phagocytosis of Staph. aureus by milk macrophages. Marco et al. (2009) worked on Growth inhibition of Staphylococcus aureus by chicken egg yolk antibodies and reported that the growth of S. aureus was inhibited by the specific IgY at concentrations of 1–5 μg/ml). Hence our main objective is to evaluate the in vitro activity of egg yolk immunoglobulin (IgY) against mastitiscausing Escherichia coli. |
| |
Material and Methods
|
| |
|
Experimental Animals
|
| |
| Twenty four week old, single comb white leghorn chickens obtained from the Abinaya Poultry Farm, Namakkal and were maintained in our animal facility. They were used in the study for the production of anti E.coli antibodies (IgY). |
| |
|
Isolation and Identification of Mastitis causing E.coli
|
| |
| Bacterial strains used in this study were isolated from milk samples from clinical cases of mastitis in cow . Milk samples were collected Tamil Nadu Agricultural University, Coimbatore. After collecting the milk sample Isolation and identification of bacteria is done on the basis of morphological, cultural and biochemical characteristics. Briefly, 0.01ml of milk is streaked on MacConkey agar and EMB agar plates to detect coliforms and gram-negative bacteria. Plates are incubated at 37°C for 48 hours. To enumerate the numbers of coliforms bacteria (E.coli) in milk, a three tube most probable number (MPN) technique was carried out. Positive tube from MPN was streaked onto Eosine Methylene Blue (EMB) agar and then incubated overnight at 35°C. Isolates were preliminarily identified as Escherichia coli by growth on an EMB agar and Gram stain (Laemmli, 1970). |
| |
|
Preparation of Antigen
|
| |
| Mastitis causing E. coli cells were grown overnight in Nutrient broth. Cells were harvested by centrifugation, washed, and resuspended in phosphate-buffered saline (PBS) at a density of 1010 cells/ml. Formaldehyde was added to a final concentration of 1% (vol/vol), and the suspension was held at 4°C for 16 h. Cells were then washed twice with PBS to remove the formaldehyde and resuspended at the same density in sterile PBS. Complete killing of the E. coli suspensions was confirmed by culture. Suspensions were stored at 4°C for before use. |
| |
|
Development of anti-E.coli antibodies in chicken
|
| |
| E.coli cells were dissolved separately in 0.9% phosphate buffered saline (PBS) in the concentration of 103 cells/ml and emulsified with Freund’s complete adjuvant (FCA) in the ratio of 1:1 using the technique of Herbert (1967). Then the solution was injected intramuscularly at the multiple sites of breast muscles of 24-week-old white leghorn chickens. Chickens received subsequent booster injections with increasing concentration of antigens at 14 days interval by the same route of administration. Test bleedings were made frequently to check the presence of anti E.coli antibodies in the serum. Eggs were collected from day 0 until the end of the experiment and stored at 4°C until testing by the indirect ELISA. |
| |
|
Purification and Characterization of anti- E.coli - antibodies from egg yolk
|
| |
| The antibodies were extracted from egg yolk by the method of Polson et al. (1980) using Polyethylene and Ammonium sulphate precipitate method. Then the content was desalted by dialysis process. The crude fraction of IgY thus obtained was further purified by DEAE cellulose ion exchange column chromatography. The IgY fraction was then concentrated with Poly Vinyl Pyrolidone (PVP) at room temperature. The protein content of the purified IgY fraction was determined by the method described by Lowry et al. (1951). The purity of Chicken egg yolk antibodies was checked by SDSPAGE. |
| |
|
Determination of antibody titer by Indirect ELISA
|
| |
| The antibody titer of the antibodies generated against E.coli was determined by indirect ELISA described by Voller et al. (1976). Nunc polysorp ELISA plates were coated with E.coli antigens using coating buffer (0.05M carbonatebicarbonate buffer, pH 9.6) and incubated at 4°C overnight. After coating plates were washed with PBST for 3 times. The empty sites blocked by 1% BSA (200 μl / well) and incubated at 37°C for 1 hour. Plates were subsequently washed and incubated with anti-E.coli- antibodies (100 μl / well). PBST and preimmune sera served as controls. Wells were washed thrice with PBST and 100 μl of diluted (1:1000) rabbit antichicken immunoglobulin coupled to Horse Radish Peroxidase (Genei Pvt Ltd, Bangalore) was added and incubated. Then the plates were washed and 100 μl of freshly prepared substrate solution (4 mg of O-phenylene diamine dissolved in 10 ml of 50 mM citrate buffer, pH 5.0 containing 10 μl of 30% hydrogen peroxide) was added. The plates were allowed to stand at room temperature in dark for 20 minutes. The reaction was stopped by adding 50 μl of 4N Sulphuric acid and plates were read at 490 nm in an ELISA reader. All samples were tested in triplicates. |
| |
|
Growth Inhibition Assay and Microscopic Slide Agglutination test
|
| |
| Growth Inhibition Assay was carried out by novel method. This experiment is to check whether the anti-E.coli IgY could inhibit E.coli growth in liquid medium. Mastitis causing E coli was inoculated into 5ml Tryptone Soya Broth (TSB), and into another 5 sets of 5 ml of TSB containing increasing concentrations of chicken egg yolk antibodies (100Nl of IgY - 500Nl of IgY) and incubated overnight at 37°C. PBS alone served as negative control and 100mg/ml of Ampicillin was the positive control. After incubation the contents were subcultured onto EMB agar and incubated overnight at 37°C. The Tubes also checked for OD and Turbidity method to check the growth inhibition of E.coli by chicken egg yolk antibodies (IgY). Antibacterial effect IgY against Mastitis causing E. coli was tested and identified by Microscopic Slide Agglutination Test (MSAT). |
| |
Results
|
| |
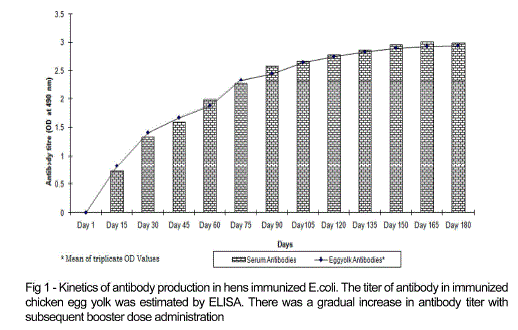
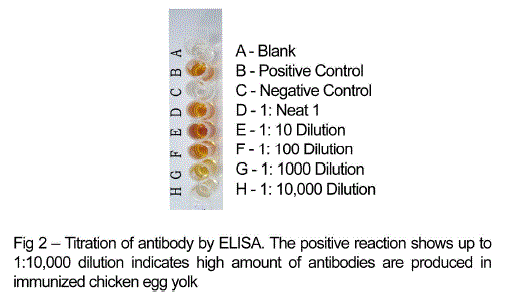
| Chicken egg yolk antibodies against mastitis causing E.coli was obtained by the method of polyethylene glycol and ammonium sulphate precipitation method from immunized chicken egg yolks and was further purified by DEAE cellulose ion exchange column chromatography. The antibody concentration of such purified fraction after each booster doses was detected by protein estimation. The IgY concentration in the egg yolk significantly increased when the chickens received booster injections at regular intervals of time. In the present study, IgY concentration varied in the range of 0.5 – 7.1mg/ml of yolk throughout the immunization period. The titre of antibodies in the immunized chicken egg yolk was carried out by ELISA. Indirect antigen capture assay (IACA) showed that the antibodies were generated in chicken against mastitis causing E.coli antigens. High peak titre of 1:10000 monovalent antibody were observed during 150th day of observation (Fig 1 and 2). |
| |
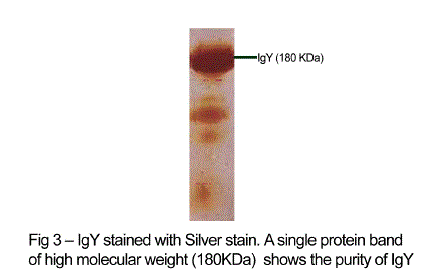
| The antibody levels of the immunized animals were significantly higher than those of the controls. This indicates that the production of specific IgY can be efficiently elicited in chickens using simple protocols of immunization and extraction. A single protein band of high molecular weight (180KDa) observed in electrophorectic analysis showed the purity of IgY (Fig 3). |
| |
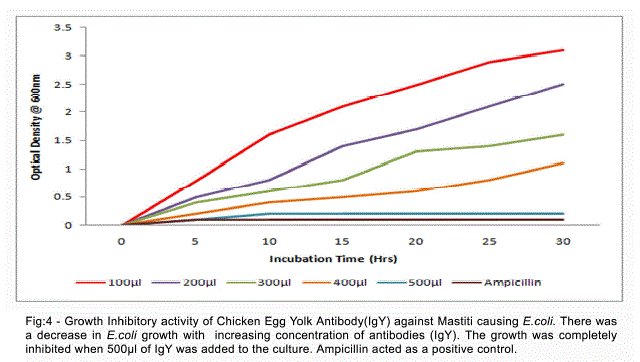
| The activity of anti-E.coli IgY was shown by the absence of growth when the specific egg yolk antibodies were added to the E.coli culture. There was a decrease in E.coli growth with increasing concentration of antibodies (IgY). The growth was completely inhibited when 500Nl of IgY was added to the culture (Fig 4). |
| |
Discussion
|
| |
| Intramammary infections of dairy cows with Gramnegative bacteria such as Escherichia coli have received a lot of attention because of their major economic impact on the dairy farm through production losses induced by an increase in somatic cell count. Escherichia coli causes inflammation of the mammary gland in dairy cows around parturition and during early lactation with striking local and sometimes severe systemic clinical symptoms. This disease affects many high producing cows in dairy herds and may cause several cases of death per year in the most severe cases. During E. coli mastitis, the host defense status is a cardinal factor determining the outcome of the disease. De and Mukharjee (2009) have been reported the overall prevalence of clinical mastitis and subclinical mastitis were 15.18% and 42.93% respectively during the month of July and August in Uttar Pradesh. Sharma (2009) had been reported prevalence of subclinical and clinical mastitis 42.18% and 10.93% respectively in dairy cows in Jammu. Early detection is essential for successful treatment. Antimicrobial therapy is pivotal for its containment and recovery. Despite the wide spread use of these drugs, antimicrobial treatment of mastitis has been less effective than desirable. Recent research showed that chicken egg yolk antibodies will act as promising alternative for diagnosis and treatment of mastitis causing organisms. The Present investigation showed that the chicken egg yolk antibodies (IgY) inhibit the growth of mastitis causing E.coli, when the specific egg yolk antibodies were added to the E.coli culture. The growth was completely inhibited when 500Nl of IgY was added to the culture. Zhen et al. (2008) reported that The specific IgY against mastitis-causing Staphylococcus aureus inhibited the growth of Staph. aureus. The growth of S. aureus was inhibited by the specific IgY at concentrations of 1–5 μg/ml (Marco et al., 2009). The yolks of eggs laid by immunized chicken have been recognized as an excellent source of polyclonal antibodies for over a decade (Polson et al., 1980; Jensenics et al., 1981). Hens therefore produce a more hygienic, cost efficient, convenient and a plentiful source of antibodies as compared to traditional method of obtaining antibodies from mammalian serum (Gassmann et al., 1990). A major advantage of using birds is that the antibodies can be harvested from the egg yolk instead of serum, thus making blood sampling obsolete. In addition, the antibody productivity of an egg-laying hen is much greater than that of a similar sized mammal (Jann Hau, 2005). |
| |
| The present experimental results indicate that the chicken egg yolk antibodies (IgY) were effective inhibiting the mastitis causing E.coli strains. The antibody generated by an alternative mean was potent enough to inhibiting the growth of E.coli. Our results show that highly purified chicken egg yolk antibodies could be used for therapy in bovine mastitis. Chicken egg yolk antibodies will play an increasing role in research, diagnostics and immunotherapy in future. |
| |
Acknowledgment
|
| |
| We thank Tamil Nadu State Council for Science and Technology (TNSCST) for providing financial Assistance through Science and Technology Projects (TNSCST/S&T Projects/RJ/VS/2010-2011). We also thank our Principal and Management for support and encouragement. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










