Keywords
Dressings; Wound; Modern wound dressings
Introduction
Wound dressing is certainly no new topic in the world of medicine. Evidence suggests that over two thousand years ago, Egyptians were already using bandages soaked with grease to manage flesh wounds [1,2]. In the two millennia that followed, newer and more effective tactics have evolved, all the while keeping two central goals in mind [3]. First, dressings should aid, or at least not interfere with the body’s intrinsic healing process. Second, dressings should protect the wound from further external damage. The most common cause of such external damage, as clinicians learned through the microscope, turns out to be infection.
These two central concepts remain the basis of moist-wound healing in the 21st century. A dressing that keeps the wound moist can contribute to more rapid healing, less agony for the patient, and better tissue integrity during recovery [4]. To prophylactically combat infection, clinicians have tried integrating various antibiotics, and more recently, metals as well as antimicrobial peptides into wound dressings [5,6]. The continual updates to dressing materials, renewed understanding of microbiology and of the physiology of healing as culminated in a greater confidence in wound treatment. Modern clinicians have countless choices in dressing the various wounds they encounter in their practices, each with their own advantages and drawbacks [7].
This review seeks to illuminate the major types of modern wound dressings and their respective applications, with a focus on their unique material and compositional characteristics. In this rapidly evolving field, it is imperative for future researchers to have an open mind and a special vision. This review hopes to inspire innovation and continual progress in the field of trauma and wound care.
Bioactive dressings
Bioactive dressings are designed to enhance wound healing in addition to being biodegradable. To better fulfill this purpose, researchers used material that are well adapted and compatible with human tissue. Common materials used include collagen and elastin, hyaluronic acid and chitosan [7]. The first three are some of the most abundant connective tissue proteins found in the human body. Chitosan is derived from chitin, a natural polysaccharide commonly found in arthropods. Chitin is extracted and deacetylated to become the more soluble chitosan, which is also cationic [8]. Like many other polysaccharides, chitin and chitosan were quickly applied to many areas of pharmacological research [9,10].
As early as the 1980s, researchers studied chitin for its use in nonwoven wound dressings [11]. There are several advantages to chitin as a dressing material, the first being its role in accelerating wound healing [12,13]. Chitin accomplishes this by adhering cut edges of skin together, tighter than fibrin glue. In addition, chitin-treated wound sites experienced more rapid granulation and subsequent epithelization. It should be noted that a more significant migration of inflammatory cells was present at chitintreated sites, with no evidence of phagocytosis of chitin itself [12]. Furthermore, chitin and quaternized chitosan-containing nanofibers were also shown to be effective at killing S. Aureus and E. Coli [14]. Electron microscopy evidence shows that these nanofibers’ antimicrobial mechanism includes hindering the adhesion of S. Aureus. Additional chemical modifications of chitin resulted in dibutyrylchitin (DBC), in an attempt to further improve biocompatibility [15]. Structural analysis of DBC revealed that it was less crystalline and had higher thermal stability than native chitin [16]. Perhaps the most important improved aspect of DBC is its high resistance to enzymatic degradation, which was studied via challenge by amylase, collagenase, and lysozymes [17]. Having both healing-enhancing properties and antimicrobial effectiveness makes chitin and its derivatives popular material candidates in modern non-woven, bioactive wound dressings. Researchers have also combined chitosan with collagen [18] and hyaluronic acid [19] in newer synthetic materials and studied their enhanced biocompatibility characteristics.
One of the most successful bioactive dressings currently available is the Aquacel Ag®, produced by ConvaTec [20]. The composition and the various properties of Aquacel serves as prime examples of the current advances in the bioactive wound dressing field and serves as a beacon that may help inspire an even better path forward. The current iteration of Aquacel is the culmination of advancements in two key technologies, Hydrofiber® and Advantage®[20]. The dressing is made of non-woven sodium carboxymethylcellulose (NaCMC) infused with ionic silver (Ag+) [20,21]. NaCMC forms the basis of the Hydrofiber® technology, as its fibers absorb wound exudate upon contact to form a moist gel, which promotes wound closure and healing [22]. It should be noted that NaCMC is also commonly used in hydrocolloid dressings [23], which will be discussed in a later section. Meanwhile, the ionic silver is combined with Ethylenediaminetetraacetic (EDTA) and Benzethonium Chloride (BEC) using the Advantage® technology, which is designed to achieve sustainable action against microbes [24].
The NaCMC fibers achieves the goal of enhancing wound healing by three major mechanisms. The first is through exudate and fluid absorption. The NaCMC fibers will form a moist gel soon after adhering to the wet surface of a wound. Importantly, the absorption of exudate is achieved vertically only, lowering the risk of maceration [25]. With traditional bandages and dressings, the moisture absorbed often spreads beyond the area of the wound. Over an extended period, the wound and the area surrounding it becomes wrinkly and edematous. This macerated state of the surrounding skin is not conducive towards healing [26]. By limiting absorption to the vertical direction, Aquacel can ensure that while the wound itself is kept moist, the surrounding tissue is kept dry and non-macerated. The left half of the figure 1 demonstrates the wide diffusion of moisture in traditional dressings, resulting in maceration and edema of surrounding tissue, hindering wound healing [26]. The right half of the figure 1 demonstrates the vertical absorption of exudate in Aquacel Ag® as a result of Hydrofiber® technology, keeping the wound site moist, minimizing maceration of normal tissue and promoting healing. Diagram not drawn to actual scale.
See Figure 1 for a demonstration of the unique vertical absorption mechanism that Aquacel employs. The second mechanism of NaCMC fibers to promote healing is through neat contouring of the wound [25]. With traditional dressings, dead space is quite commonly seen between the wound and the dressing fibers. Fluid and tissue debris that accumulate in this dead space creates the perfect environment for microbes to thrive, causing infections [27]. Aquacel’s neat contouring to the wound surface helps minimize dead space and thus ensure a smoother, uncomplicated recovery process. The last mechanism of NaCMC fibers in enhancing healing lies in the dressing removal process. The gellike nature of the Hydrofibers® minimally stick to the granulation tissue of the wound, thus preventing secondary damage common in the removal of traditional dressings [28].
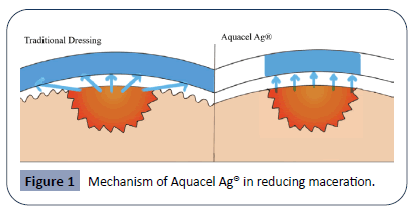
Figure 1: Mechanism of Aquacel Ag® in reducing maceration.
The strategy that Aquacel employs to combat bacteria is ionic silver, which has long been proven to be an effective antimicrobial agent [29]. Silver does so through several mechanisms, including binding to bacterial DNA to prevent cell division, blocking nutrient transport and inhibiting energy production at the bacterial cell wall [30]. In Aquacel, ionic silver is compounded with EDTA and BEC, which enhances silver’s antimicrobial efficacy [24,31]. EDTA is often used to enhance antibiotic effects via prevention of bacterial aggregates and promoting the access of antimicrobials to the bacterium [32]. BEC reduces the surface tension of the dressing-wound gel interface, allowing EDTA and ionic silver better access to bacteria [33]. Aquacel compounds ionic silver with EDTA and BEC using Advantage® technology to achieve great efficacy against microbes, even Pseudomonas biofilms, commonly seen in nosocomial infections [34].
Aquacel can act as a great benchmark for the future development of bioactive wound dressings. Two central development strategies will likely still revolve around actively promoting wound healing and preventing infection. Aquacel is based on NaCMC, but future bioactive dressings may choose to employ chitin, collagen or elastin as adjunct in a new synthetic material [35]. If these components are indeed compatible, the ideal dressing would combine the controlled vertical absorption of NaCMC fibers with the greater biocompatibility of chitin and collagen. In terms of antimicrobial considerations, there are even more possibilities and opportunities. In addition to prophylactic antibiotics and silver nanoparticles, antimicrobial peptides are an interesting direction to explore [36]. In the setting of rapidly developing drug resistance among bacteria, treatment methods like antimicrobial peptides that can circumvent this resistance will be promising. Certain studies have already began exploring possibilities of incorporating these peptides into wound dressings among other applications [37,38].
Hydrocolloid dressings
A hydrocolloid (HCD) is a two layered dressing surface that is opaque, transparent, biodegradable, and breathable. It can adhere over a wound with no separate taping to promote healing as a primary or secondary dressing [39]. The inner layer consists of self-adhesive gel-forming carboxymethylcellulose polymer, pectin, gelatin or an elastomer. The outer layer consists of polyurethane film that seals the wound to protect it from bacteria, foreign debris, and shearing [40,41]. This outer layer can either be occlusive or semi occlusive [42]. There are many types of HCD dressings. The fibrous type is the most popular and composed of sodium carboxymethylcellulose which form to become a gel when it comes into contact with any fluid [41]. Shapes and sizes vary for HCDs and they also are presented in paste, powder, or granule form [40].
In general, the mechanism of HCDs in wound healing is effective because the inner layer provides a moist environment, which promotes autolytic debridement, and the outer layer prevents contamination from bacteria and fluids. First, the HCD dressing is placed in contact with the wound. As the wound produces exudate, the inner layer absorbs the fluids and swells due to increased oncotic pressure to form a gel-like matrix structure. The retention of moisture facilitates an acidic environment to inhibit bacterial growth. Moreover, the inner layer pushes down on the wound, thus promoting fibrinolysis, angiogenesis, and healing without breaking down or softening the patient’s skin. Furthermore, the exudate that is absorbed will exert a pressure back onto the wound to reduce further exudate production. See Figure 2 for a simplified demonstration of this mechanism. The outer layer is speculated to play a role in trapping white blood cells that liquefy and prevent necrosis [39,40,42-45].
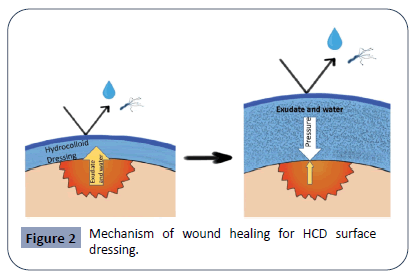
Figure 2: Mechanism of wound healing for HCD surface dressing.
Indications of using HCDs include healing of partial and full thickness acute or chronic wounds by promoting autolytic debridement [42]. These wounds can include high friction areas (i.e. sacrum, heels) and prevent device-related pressure injuries in intubated ICU patients [42,46]. Although HCDs assume the shape of whatever they are placed onto, they should still be used in line and not to fill a wound cavity. This is because the HCD dressing may strip peri-wound skin if not changed frequently [44]. It is not recommended to use HCDs for healing of dry wounds or wounds with heavy exudation [40,42]. If a patient were to receive HCD dressing for a wound with excess exudate, it is important to remove the HCD prior to the occurrence of hypergranulation [47].
Reviewing literature suggests that the application of HCD dressings were most commonly associated with treatment of ulcers, but may also be beneficial for burns, skin donor sites, surgical/traumatic wounds, and pediatric/neonatal wounds. Some literature concludes that HCD is far superior to other conventional gauze dressings in healing ulcers [48-53]. However, other studies found no statistical significance between dressings in healing ulcers [54-57]. For burn treatment, HCD should be used for superficial burns without necrosis [58]. For skin donor sites, HCD offered better cosmetic results and accelerated healing rates compared to those of conventional dressing [59]. In addition, HCD can reduce pain and heal surgical and traumatic wounds faster than conventional dressing can [60]. Finally, for pediatric/ neonatal wounds, HCD is excellent for protecting pediatric skin and can mold into patterns suitable for smaller neonatal sizes [61,62].
Hydrogel dressings
Hydrogel wound dressings are hydrophilic polymers crosslinked by in-situ processes such as electrical fields, magnetic fields, temperature shift, pH shift, light intensity shift, radiation, addition of solvent, and shift in pressure [63,64]. Addition and removal of these processes causes a volumetric shift in the hydrogel, which can swell and reversibly compress back to original size [63]. They are highly flexible dressings with a water content similar to that of the body’s own soft tissues. The outer mesh of hydrogel dressings prevents microbial entry. Hydrogels serve as beneficial wound dressings because they control water loss from the wound, keep the wound moist, exchange gases with the atmosphere, are similar in consistency to soft tissue, and can be removed more easily than alternative dressings due to their non-adherent nature [64]. Hydrogels are set apart from alternative wound dressings by their 60-78% water capacity, which can rehydrate wounds to aid in autolytic debridement without the need for significant exudate release to maintain a gel composition [65-67]. Because they absorb only 14-28% of moisture from the wound, hydrogel dressings perform best when placed on lightly exuding or dry wounds, such as radiation wounds, ulcers, post-surgical incisions, a wide variety of burns, and even meningococcal purpuric macules [66,68]. Hydrogels can absorb some exudate; they also aid in fibroblast proliferation and keratinocyte migration in the wound site [64].
However, many hydrogels suffer from a low mechanical stability due to a tradeoff of viscosity for easy application and elasticity for continued flow after initial application [69]. Because of this tradeoff, as well as inconsistencies in macromolecular structure and low friction between polymer chains, Hydrogels tend to leak more than alternative wound dressings [70]. Water capacity, flexibility, and mechanical stability are major points of differentiation between extant hydrogels on the market [6]. Many hydrogels have improved their stability through an innovative composition. Double network hydrogels are a more stable gel which overlaps two distinct polymer networks, which can synergize to enhance factors like stability, tensile strength, and biocompatibility [71].
Since their invention by Wichterle and Lim in 1960, hydrogel composition has evolved to increase stability and to function as an effective delivery mechanism [72]. Poly (vinyl alcohol)- hydrogel hybrids, which combine natural and synthetic polymers, are very common on the market [73]. Additionally, chitosan bioactive dressings and alginates, which are often utilized in hydrogels, are becoming increasingly popular. Manufacturers are moving away from propylene glycol, which can be a mildly irritating to certain patients [66]. Because the macromolecular network of hydrogel composition can replicate the extra-cellular matrix, biological components or antibiotics can be paired with hydrogels to be delivered to the wound in a time-release manner. Drugs can be added during the gelation process via entrapment, covalent linkage, or use of a micelle/liposome carrier [74]. As the hydrogel swells with moisture and exudate, or as a stimulus is applied, drugs are released from the gel into the wound site, as shown in Figure 3. The hydrophobic polymer chains (green) with cross-linkages (red) undergoes gelation with the drug (blue), which becomes entrapped in the hydrogel. The bottom half of the figure demonstrates three delivery mechanisms from left to right: Stimulus-mediated drug release, simple diffusion through the mesh and swelling release. The first mechanism involves a stimulus (magnetism, glucose, enzymes, electricity, light, radiation, pH, ultrasound, or temperature) applied to the hydrogel, which then promotes drug release. The second depiction shows the drug as it slowly diffuses out of the polymer chains in a time-release format. The last mechanism involves the hydrogel swelling with moisture and exudate from the wound. The influx of water then flushes out the drug from the now loosened polymer chains. This can be particularly beneficial in increasing patient compliance, thus improving therapeutic outcomes for those with chronic wounds [75]. With this advantage, hydrogel dressings can be further modified to be an effective mechanism for delivery of drugs, nanoparticles, enzymes, stem cells, or growth factors [76-78]. Because of this addition mechanism, some modern hydrogels such as DermaGel and Intrasite Gel have been proven to have a higher efficacy against Candida, Pseudomonas, and Staphylococcus infections than traditional dressings [66]. Other hydrogels even have enzymatic mechanisms built-in to fight antibiotic resistance in bacteria [79,80].
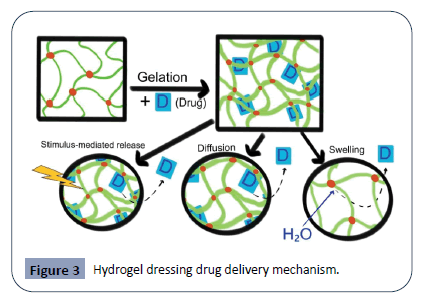
Figure 3: Hydrogel dressing drug delivery mechanism.
Alginate dressings
Alginate is another biopolymer frequently used in wound dressings. Derived from the cell walls of brown algae species (Ascophyllum nodosum, Laminaria Hyperborean, Macrocystis pyrifera, Laminaria japonica, Laminaria digitate) as well as from some bacteria (Azotobacter vinelandii, Pseudomonas spp.) [81], alginate is a linear polysaccharide composed of β-(1,4) linked D-mannuronic acid (M) residues and α-(1,4)-linked L-guluronic acid (G) residues [82]. Alginate monomers are arranged of consecutive M-residues (M-Blocks), G-residues (G-Blocks), or alternating M and G residues (MG-Blocks) depending its source material [82]. The unique arrangement of these blocks is demonstrated in Figure 4 and determines the physical and biomechanical characteristics of the alginate polymer. The left side of the image illustrates the egg-box structure that forms from the binding of G-residues on opposite sides of the alginate polymer in the presence of divalent cations. This crosslinking of alginate polymers leads to the formation of alginate gels. The right side of the image illustrates the structure of M-Blocks, G-Blocks, and M-G Blocks that make up alginate monomers and give alginate its biologic and mechanical properties [82]. Increasing the M:G ratio stimulates cytokine production, leading to a greater immunogenic effect. Decreasing this ratio leads to a stiffer, more stable structure [83]. The monomeric units come together to form linear, unbranched polymers of alginate, which can then crosslink with divalent cations (Ca2+ and Ba2+) via ionic bonding to form alginate gels. In this process, G-residues on opposite sides of the polymer bind to each other, forming a diamond shaped “eggbox” structure with a hydrophilic center that binds the divalent cations.
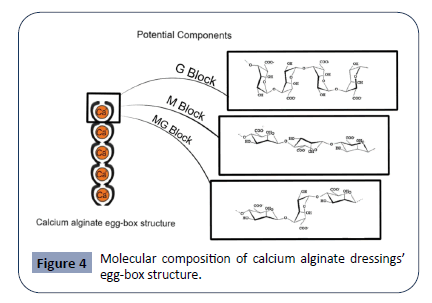
Figure 4: Molecular composition of calcium alginate dressings’ egg-box structure.
Alginate dressings are formed from polymers of alginate coated in calcium and sodium salts [84], and ionic exchange between the dressing and wound exudate leads to crosslinking and the formation of alginate hydrogels [85]. Alginate is well suited for wound care because its features resemble those of the human tissue extracellular matrix while being naturally biocompatible [86], non-immunogenic [85] (if properly purified [83]), and affordable [87]. The strongly hydrophilic nature of alginate increases its capacity for wound exudate absorption (absorbs 15–20 times its weight in fluid) [88]. It provides the wound with a moist microenvironment to promote healing [89], and confers protection against bacteria [90,91]. The high absorption capacity makes these types of dressings useful for minimally, moderately, and heavily exudative wounds [92-96] as well as chronic wounds [97,98]. The release of calcium ions in alginate dressings activates platelets, improving alginate’s hemostatic capabilities and making it effective for the management of bleeding wounds [99,100]. These biological and chemical properties of alginate work together to increase the efficacy of wound healing [90,101].
Different nanomaterials can also be added to alginate gels to increase their antimicrobial capabilities or to promote tissue growth. Alginate has been combined with nano zinc oxide [102], silver nanoparticles [103,104], ammonium salts [105], chitosan [106], or loaded with antibiotics such as moxifloxacin [107] and ampicillin [108]. Simvastatin-incorporated alginate has shown an ability to upregulate hypoxia-inducible factor-1α and vascular endothelial growth factor, leading to increased angiogenesis [109]. Amniotic fluid loaded into alginate led to greater wound healing by increasing cell proliferation and spreading. It also led to a greater degree of collagen secretion at the wound site [110].
Despite is many advantages, alginates’ relatively poor mechanical properties [111,112] necessitate that they be combined with synthetic and natural polymers to improve their stability [113,114]. One ionic polymer that is frequently combined with alginate is chitosan, and ionic crosslinking between the two polymers leads to increased hydrogel structural stability [97,115-118] as well as stronger antimicrobial properties [106,119]. Alginate has also been combined with gelatin [120-122], nanocellulose [123], bioglass/agarose [124], carboxymethyl chitosan [125,126], and polyacrylamide [127] to improve its mechanical stability. Another biomechanical limitation of alginate is that higher molecular weight alginate strands cannot be broken down due to a lack of mammalian alginate-degrading enzymes. Oxidizing the M and G residues of alginate has been shown to increase the biodegradability of alginate [128].
While alginate hydrogels are the most commonly used form of alginate wound dressing, additional forms of alginate-based dressings include foams, wafers, films, membranes, nanofibers, and sponges [85]. All these different forms of alginate contain its innate properties of biocompatibility, non-immunogenicity, and high absorption capacity. Alginate foams are also easy to apply, with minimal discomfort to the patient and conveniently removable from the wound site [129]. They also have an extended hydration time, large surface area, a high degree of porosity, and can be loaded with bioactive agents [130]. Freeze-drying alginate polymer solutions via the lyophilization method results in solid, porous wafers whose structure resembles foam dressings but turn into a gel upon coming into contact with wound exudate [131,132]. Alginate based films and membranes can be used as dressings, but are not effective for wounds with high levels of exudate [7,99]. Nanofibers have shown some potential as an alginate-based wound dressing due to their ability to improve epithelial cell proliferation and tissue formation [35], promote hemostasis [133], and their strong biomechanical [134] and antimicrobial [135,136] properties, but they are currently both difficult and expensive to produce [137,138].
The future of alginate dressings is likely going to focus on testing the loading and releasing of various bioactive molecules to wound sites to better optimize the dressing’s tissue repair and antimicrobial effects. Currently, alginates loaded with a single antibiotic show much faster rates of drug delivery than dualantibiotic loaded alginate dressings, but microfluidic technology could produce pH-responsive alginate composites with a greater ability to deliver dual-antibiotics [139]. Modification of alginate to be more biomechanically stable and biodegradable while maintaining its absorptive capabilities will also likely be a key area of focus in the bioengineering of novel alginate dressings [89]. Lastly, if the clinical effectiveness of alternative forms of alginate dressings can be increased while simultaneously decreasing their production cost, their usage may become significantly more widespread [85].
Semi-permeable film dressings
Semi-permeable film dressings are non-porous, flexible, thin, and transparent, sheets of polyurethane covered with an adhesive layer that allows the dressing to adhere to the skin. These polyurethane sheets are permeable to gas such as O2 and CO2, but impermeable to liquid and microbial organisms. Semipermeable dressings prevent microbial migration and protect the wound. They are intended for simple superficial injuries such as lacerations, burns and abrasions.
As a flexible sheet, semi-permeable film dressings: (i) easily adhere to the skin, (ii) allow for evaporation of moisture, (iii) relieve pain, (iv)act as barriers from the external environment (v) allow for easy inspection of wound without dressing removal [140]. In terms of disadvantages, semi-permeable films can cause injuries on removal and can pool exudate on the wound when used as a secondary dressing. Furthermore, semipermeable films are non-absorptive dressings, and inappropriate dressing choices can lead to the damage of the surrounding skin and, increasing risk of infection.
Semi-permeable films can be used as primary dressing or secondary dressings when applied simultaneously over an exudate, such as a foam. When applied as a secondary dressing, semi-permeable films act as a protective cover for the wound. In surgery, semipermeable films can be used to protect postoperative wounds and have been more cost effective and efficient compared to traditional gauze dressing [84]. The use of semi-permeable films serves as a barrier to external contamination. Further, this barrier prevents microbials entrance and infection. As a result, the wound can easily self-heal without the infringement of external factors. Additionally, semipermeable film dressings are beneficial in preventing and managing of radiation-induced skin reactions, such as radiation dermatitis of different grades [141].
Some semi-permeable film dressings currently on market are Biofilm, Biooclusive, Hydrofilm, Mepilex Film, OpSite, OpSite Flexifix Gentle and Tegaderm [142]. These products are used for light abrasions or postoperative sutured wounds. Films can be maintained in place for up to seven days, and the replacement period may depend on the size and type of the wound. A future enhancement of semi-permeable film dressing is an ozone generating system film dressing [143]. Wearable and flexible ozone (O3) generating system has been suggested to treat non-healing and critically infected wounds by providing strong antibacterial properties while accelerating local tissue regeneration. Ozone is known to inactivate bacteria, viruses, fungi, yeast, and protozoa through the oxidation of phospholipids and lipoproteins in the cell envelope, which can weaken or destroy bacterial walls [143]. Further, the presence of ozone activates fibroblast growth factors, triggers angiogenesis, and promotes tissue regeneration [144]. The ozone generating system proposes a multilayered patch that utilizes the characteristics of ozone against microbials and treat chronically infected wounds. This system works by uniformly dispersing a small amount of ozone to the site of infection or wound via a portable device, which is demonstrated in Figure 5. This figure demonstrates the various layers of the ozone-enhanced wound dressing system as outlined by Roth et al. PDMS is the abbreviation for Polydimethylsiloxane, an organosilicon compound that confers hydrophobicity. This patch thus incorporates a hydrophobic and highly ozonepermeable outer layer and an inner dispersion layer for more a more uniformed gas distribution [143]. Figure is not drawn to actual scale.
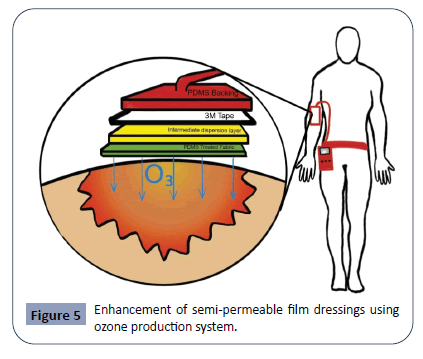
Figure 5: Enhancement of semi-permeable film dressings using ozone production system.
Research has shown that a small amount of ozone is cytotoxic to antimicrobial resistant strains bacteria, P. aeruginosa and S. epidermidis, but noncytotoxic to human basal skin cells [143]. However, a drawback of such a system is that it requires a sizable portable device that is only likely to be available in clinic settings [143]. In prospect, the development of a portable ozone semipermeable film would significantly enhance the management of chronically infected wounds against resistant microbes.
Another notable approach to semi-permeable films would be incorporating it with nanomaterials-based film dressings [84]. Nanomaterials (NMs) can be designed to have antibacterial, anti-inflammatory, proangiogenic, and proliferative properties [84]. Additionally, NMs can modulate the expression of essential proteins and signal molecules to improve wound healing processes. Research has proposed that a nanofiber-based semipermeable film dressing can be used at the site of injury. Nanofibrous membranes can detect changes in pH of an injured site and release antibiotics and other drugs to enhance wound healing.
Concluding Remarks and Outlook
Understanding the subtle differences between wound dressings can potentially help the clinician achieve a better outcome for his/her patient, whether that is a less painful recovery, a shorter hospital stay, or in the most emergent of cases, decreased mortality [145]. However, it is also impossible for every practicing physician to memorize information about every type of wound dressing available in the market. Thus, this review aims to inform clinicians about the most significant aspects of each major category of trauma dressing. It goes into detail regarding their material and compositional characteristics for those that are more concerned.
The preeminent goal in the development of trauma dressings would be to create one that is highly adaptable and effective for the widest range of wound conditions. For example, combining the biocompatibility of bioactive dressings with the non-irritant properties of hydrogels and exudate-suppressing qualities of the hydrocolloids [7,12,146]. This may be impossible at present due to the inherent limits of material synthesis technology, but may become a reality soon, with the advent of novel methods of creating more complex composites. Currently, most advancement in the field comes from discoveries of new materials that enhance healing or inhibit microbial activity [43]. This serves as a solid foundation for the next significant phase in wound dressing research- the mixing and matching of such materials in search for the most efficacious composite or synthetic. Given the dire current situation regarding the Covid-19 pandemic [147-149], investments in the biotech field is surging, helping to accelerate microbiology research in the near future.
There will be tense competition in the arena of future trauma dressing research. However, this competition can be “synergistic” and help the industry flourish. The copious number of materials studied now can will translate to countless future opportunities. Creation of newer, better and more affordable wound dressings will benefit millions of patients around the world and perhaps even revolutionize acute trauma care.
Acknowledgements
We would greatly acknowledge the supports from Shenzhen Science and Technology Program (Grant No: KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology.
Conflict of Interests
The authors have no conflict of interests to declare.
28591
References
- Queen D, Orsted H, Sanada H, Sussman G (2004) A dressing history. International wound journal 1: 59-77.
- Uzun M, Anand SC, Shah T (2013) In vitro characterisation and evaluation of different types of wound dressing materials. J Biomed Eng Technol 1: 1-7.
- Forrest RD (1982) Early history of wound treatment. J R Soc Med 75: 198-205.
- Foertsch CE, O'Hara MW, Stoddard FJ, Kealey GP(1998) Treatment-resistant pain and distress during pediatric burn-dressing changes. J Burn Care Rehabil 19: 219-224.
- Broughton G, Janis JE, Attinger CE (2006) A brief history of wound care. Plast Reconstr Surg 117: 6S-11S.
- Castellano JJ, Shafii SM, Ko F, Donate G, Wright TE, et al. (2007) Comparative evaluation of silverâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂcontaining antimicrobial dressings and drugs. Int Wound J 4: 114-122.
- Dhivya S, Padma VV, Santhini E (2015) Wound dressings - a review. BioMedicine 5: 22.
- Rinaudo M (2006) Chitin and chitosan: properties and applications. Progress in polymer science 31: 603-632.
- Dodane V, Vilivalam VD (1998) Pharmaceutical applications of chitosan. Pharmaceutical Science & Technology Today 1: 246-253.
- Zhang Y, Sun L (2020) Sweetening the Deal: Glycosylation and its Clinical Applications. Journal of Biomedical Sciences 9: 3-9.
- Ohshima Y, Nishino K, Okuda R, Minami A, Kihune K (1987) Clinical application of chitin non-woven fabric as wound dressing. European Journal of Plastic Surgery 14: 207-211.
- Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, et al. (2002) Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 23: 833-840.
- Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, et al. (2003) Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 24: 3437-3444.
- Ignatova M, Manolova N, Markova N, Rashkov I (2009) Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol Biosci 9: 102-11.
- Chilarski, A, Szosland L, KruciÅÃÂÃÂââ¬Ã
¡ÃÂâÂÂââ¬Ã
¾ska I, Kiekens P, BÅÃÂÃÂââ¬Ã
¡ÃÂâÂÂââ¬Ã
¡asinska A, et al. (2007) Novel dressing materials accelerating wound healing made from dibutyrylchitin. Fibres & Textiles in Eastern Europe 15: 77-81.
- Wan Y, Creber KAM, Peppley B, Bui VT (2004) Structure and ionic conductivity of a series of diâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂoâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂbutyrylchitosan membranes. Journal of applied polymer science 94: 2309-2323.
- Muzzarelli RAA, Guerrieri M, Goteri G, Muzzarelli C, Armeni T, et al. (2005) The biocompatibility of dibutyryl chitin in the context of wound dressings. Biomaterials 26: 5844-5854.
- Wang CC, Chen CC (2005) AntiâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂbacterial and swelling properties of acrylic acid grafted and collagen/chitosan immobilized polypropylene nonâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂwoven fabrics. Journal of applied polymer science 98: 391-400.
- Fahmy HM, Aly AA, Abou-Okeil A (2018) A non-woven fabric wound dressing containing layer - by - layer deposited hyaluronic acid and chitosan. Int J Biol Macromol 114: 929-934.
- Barnea Y, Weiss J, Gur E (2010) A review of the applications of the hydrofiber dressing with silver (Aquacel Ag®) in wound care. Ther Clin Risk Manag 6: 21-27.
- Williams C (1999) An investigation of the benefits of Aquacel Hydrofibre wound dressing. Br J Nurs 8: 676-7.
- Robinson B (2000) The use of a hydrofibre dressing in wound management. Journal of wound care 9: 32-34.
- Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydrate polymers 72: 43-51.
- Hurlow J (2012) AQUACEL® Ag dressing with Hydrofiber® technology. Advances in wound care 1: 104-107.
- Mishra A, Whitaker IS, Potokar TS, Dickson WA (2007) The use of aquacel Ag® in the treatment of partial thickness burns: A national study. Burns 33: 679-680.
- Cutting K, White R (2002) Maceration of the skin and wound bed 1: its nature and causes. Journal of wound care 11: 275-278.
- Cutting K, White R, Hoekstra H (2009) Topical silverâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂimpregnated dressings and the importance of the dressing technology. Int Wound J 6: 396-402.
- Kumar R, Münstedt H (2005) Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 26: 2081-2088.
- Kim JS, Kuk E, Yu KN, Kim J, Park SJ, et al. (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine 3: 95-101.
- Kolera D (2005) A silver tale: pseudomonas vs Aquacel Ag. Primary Intention: The Australian Journal of Wound Management 13: 181-182.
- Lambert R, Hanlon G, Denyer SP (2004) The synergistic effect of EDTA/antimicrobial combinations on Pseudomonas aeruginosa. Journal of applied microbiology 96: 244-253.
- Shintre MS, Gaonkar TA, Modak SM (2006) Efficacy of an alcohol-based healthcare hand rub containing synergistic combination of farnesol and benzethonium chloride. International journal of hygiene and environmental health 209: 477-487.
- Bjarnsholt T, Kirketerp-Møller K, Kristiansen S, Phipps R, Nielsen AK, et al. (2007) Silver against Pseudomonas aeruginosa biofilms. APMIS 115: 921-928.
- Abrigo M, McArthur SL, Kingshott P (2014) Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci 14: 772-792.
- Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415: 389-395.
- Felgueiras HP, Amorim MTP (2017) Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf B Biointerfaces 156: 133-148.
- Gomes AP, Mano JF, Queiroz JA, Gouveia IC (2015) Incorporation of antimicrobial peptides on functionalized cotton gauzes for medical applications. Carbohydrate polymers 127: 451-461.
- Thomas S (1997) A comparative study of the properties of twelve hydrocolloid dressings. World Wide Wounds.
- Morin RJ, Tomaselli NL (2007) Interactive dressings and topical agents. Clin Plast Surg 34: 643-658.
- Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T (2014) Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev.
- Weller CD, Team V, Sussman G (2020) First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front Pharmacol 11: 155.
- Ovington LG (2007) Advances in wound dressings. Clin Dermatol 25: 33-38.
- Ovington LG (2001) Wound care products: how to choose. Home Healthc Nurse 19: 224-231.
- Vowden K, Vowden P (2017) Wound dressings: principles and practice. Surgery 35: 489-494.
- Dumville JC, Deshpande S, O'Meara S, Speak K (2012) Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev.
- Swanson T (2020) Modern dressings and technologies. Wound Management for the Advanced Practitioner.
- Singh A, Halder S, Menon GR, Chumber S, Misra MC, et al. (2004) Meta-analysis of randomized controlled trials on hydrocolloid occlusive dressing versus conventional gauze dressing in the healing of chronic wounds. Asian J Surg 27: 326-332.
- Bouza C, Saz Z, Muñoz A, Amate JM (2005) Efficacy of advanced dressings in the treatment of pressure ulcers: a systematic review. J Wound Care 14: 193-199.
- Chaby G, Senet P, Vaneau M, Martel P, Guillaume J, et al. (2007) Dressings for acute and chronic wounds: a systematic review. Arch Dermatol 143: 1297-1304
- Zheng X, Li J (2015) Comparison of the treatment of hydrocolloid and saline gauze for pressure ulcer: A meta-analysis of randomized controlled trials. Int J Clin Exp Med 8: 20869-20875.
- Tolentino AC, Dick S, Amaya R (2016) Analysis of two pressure ulcer dressings for cost reduction in the Brazilian public health system. Value in Health A844.
- Cai JY, Zha ML, Chen HL (2019)Use of a Hydrocolloid Dressing in the Prevention of Device-related Pressure Ulcers During Noninvasive Ventilation: A Meta-analysis of Randomized Controlled Trials. Wound Manag Prev 65: 30-38.
- Vermeulen H, Ubbink DT, Goossens A, de Vos R, Legemate DA (2005) Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg 92: 665-672.
- Palfreyman SJ, Nelson EA, Lochiel R, Michaels JA (2006) Dressings for healing venous leg ulcers. Cochrane Database Syst Rev pp: Cd001103.
- Walter CJ, Dumville JC, Sharp CA, Page T (2012) A cochrane systematic review and meta-analysis examining the role of wound dressings on surgical site infection rates. Colorectal Disease 13: 83-84.
- Wu L, Norman G, Dumville JC, O'Meara S, Bell-Syer SEM (2015) Dressings for treating foot ulcers in people with diabetes: An overview of systematic reviews. Cochrane Database Syst Rev pp: CD010471.
- Smith DJ, Thomson PD, Garner WL, Rodriguez JL (1994) Burn wounds: infection and healing. Am J Surg 167: 46S-48S.
- Doherty C, Lynch G, Noble S (1986) Granuflex hydrocolloid as a donor site dressing. Care Crit III 2: 193-194.
- Thomas S (2008) Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J 5: 602-613.
- Steen EH, Wang X, Boochoon KS, Ewing DC, Strang HE, et al. (2020) Wound Healing and Wound Care in Neonates: Current Therapies and Novel Options. Adv Skin Wound Care 33: 294-300.
- Taquino LT (2000) Promoting wound healing in the neonatal setting: process versus protocol. J Perinat Neonatal Nurs 14: 104-118.
- Bahram M, Mohseni N, Moghtader M (2016) An introduction to hydrogels and some recent applications. Emerging concepts in analysis and applications of hydrogels. IntechOpen.
- Kamoun EA, Kenawy ERS, Chen X (2017) A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. Journal of Advanced Research 8: 217-233.
- Sprung P, Hou Z, Ladin DA (1998) Hydrogels and hydrocolloids: an objective product comparison. Ostomy Wound Management 44: 36-42.
- Jones A, Vaughan D (2005) Hydrogel dressings in the management of a variety of wound types: A review. Journal of Orthopaedic Nursing 9: S1-S11.
- Vernon T (2000) Intrasite Gel and Intrasite Conformable: the hydrogel range. British Journal of Community Nursing 5: 511-516.
- Thomas S, Humphreys J, Fear-Price M (1998) The role of moist wound healing in the management of meningococcal skin lesions. Journal of wound care 7: 503-507.
- Li J, Suo Z, Vlassak JJ (2014) Stiff, strong, and tough hydrogels with good chemical stability. Journal of Materials Chemistry B 2: 6708-6713.
- Lukaszczyk J (1995) Investigations on preparation and properties of modified polyacrylamide hydrogels for application as wound dressing materials. Polim Med 25: 15-23.
- Gong JP, Katsuyama Y, Kurokawa T, Osada Y (2003) DoubleâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂnetwork hydrogels with extremely high mechanical strength. Advanced Materials 15: 1155-1158.
- Wichterle O, Lim D (1960) Hydrophilic gels for biological use. Nature 185: 117-118.
- Jiang S, Liu S, Feng W (2011) PVA hydrogel properties for biomedical application. Journal of the Mechanical Behavior of Biomedical Materials 4: 1228-1233.
- Liu H, Wang C, Li C, Qin Y, Wang Z, et al. (2018) A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC advances 8: 7533-7549.
- Li J, Mooney DJ (2016) Designing hydrogels for controlled drug delivery. Nature Reviews Materials 1: 1-17.
- Toh WS, Lee EH, Guo XM, Chan JKY, Yeow CH, et al. (2010) Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials 31: 6968-6980.
- Yamamoto M, Ikada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel. Journal of Biomaterials Science, Polymer Edition 12: 77-88.
- Huang G, Gao J, Hu Z, John JV, Ponder BC, et al. (2004) Controlled drug release from hydrogel nanoparticle networks. J Control Release 94: 303-311.
- Ng VWL, Chan JMW, Sardon H, Ono RJ, García JM, et al. (2014) Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv Drug Deliv Rev 78: 46-62.
- Li W, Dong K, Ren J, Qu X (2016) A βâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂLactamaseâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂImprinted Responsive Hydrogel for the Treatment of AntibioticâÃÂÃÂââ¬Ã
¡ÃÂâÂÂââÂÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂResistant Bacteria. Angewandte Chemie 128: 8181-8185.
- Smidsrød O, Skjåk-Braek G (1990) Alginate as immobilization matrix for cells. Trends Biotechnol 8: 71-78.
- Ching SH, Bansal N, Bhandari B (2017) Alginate gel particles-A review of production techniques and physical properties. Crit Rev Food Sci Nutr 57: 1133-1152.
- Szekalska M, PuciÅÃÂÃÂââ¬Ã
¡ÃÂâÂÂââ¬Ã
¡owska A, SzymaÅÃÂÃÂââ¬Ã
¡ÃÂâÂÂââ¬Ã
¾ska E, Ciosek P, Winnicka K (2016) Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. International Journal of Polymer Science 2016: 7697031.
- Stoica AE, Chircov C, Grumezescu AM (2020) Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 25.
- Aderibigbe BA, Buyana B (2018) Alginate in Wound Dressings. Pharmaceutics 10: 42.
- WawrzyÅÃÂÃÂââ¬Ã
¡ÃÂâÂÂââ¬Ã
¾ska E, Kubies D (2018) Alginate matrices for protein delivery - a short review. Physiol Res 67: S319-s334.
- Parkes J (2015) A clinical in-market evaluation of an alginate fibre dressing. Br J Nurs 24: S28, s30-5.
- Rastogi P, Kandasubramanian B (2019) Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 11: 042001.
- Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku RE (2020) Alginate-based composite materials for wound dressing application: A mini review. Carbohydrate Polymers 236: 116025.
- Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37: 106-126.
- Weir D (2020) Local Wound Care for Dermatologists. Editors: Alavi A, Maibach HI (Editors). Springer International Publishing: Cham, pp: 25-34.
- Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T (2016) Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev pp: Cd003091.
- Dumville JC, Keogh SJ, Walker RM (2015) Alginate dressings for treating pressure ulcers. Cochrane Database Syst Rev pp: Cd011277.
- Dumville JC, O'Meara S, Deshpande S, Speak K (2012) Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev pp: CD009110.
- O'Meara S, James MM, Adderley UJ (2015) Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2015: Cd010182.
- Saco M, Howe N, Nathoo R, Cherpelis B(2016) Comparing the efficacies of alginate, foam, hydrocolloid, hydrofiber, and hydrogel dressings in the management of diabetic foot ulcers and venous leg ulcers: a systematic review and meta-analysis examining how to dress for success. Dermatol Online J 22.
- Straccia MC, d'Ayala GG, Romano I, Oliva A, Laurienzo P (2015) Alginate hydrogels coated with chitosan for wound dressing. Mar Drugs 13: 2890-908.
- Ziegler UE, Schmidt K, Keller HP, Thiede A (2003) Treatment of chronic wounds with an alginate dressing containing calcium zinc and manganese. Fortschr Med Orig 121: 19-26.
- Sood A, Granick MS, Tomaselli NL (2014) Wound Dressings and Comparative Effectiveness Data. Adv Wound Care (New Rochelle) 3: 511-529.
- Yang Q, Lei S (2015) Alginate Dressing Application in Hemostasis After Using Seldinger Peripherally Inserted Central Venous Catheter in Tumor Patients. Indian J Hematol Blood Transfus 31: 434-438.
- Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J (2013) The local treatment and available dressings designed for chronic wounds. J Am Acad Dermatol 68: e117-e126.
- Mohandas A, Kumar PTS, Raja B, Lakshmanan VK, Jayakumar R (2015) Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int J Nanomedicine 10: 53-66.
- Singh R, Singh D (2012) Radiation synthesis of PVP/alginate hydrogel containing nanosilver as wound dressing. J Mater Sci Mater Med 23: 2649-2658.
- Munhoz DR, Bernardo MP, Malafatti JOD, Moreira FKV, Mattoso LHC (2019) Alginate films functionalized with silver sulfadiazine-loaded [Mg-Al] layered double hydroxide as antimicrobial wound dressing. Int J Biol Macromol 141: 504-510.
- Zare-Gachi M, Daemi H, Mohammadi J, Baei P, Bazgir F, et al. (2020) Improving anti-hemolytic, antibacterial and wound healing properties of alginate fibrous wound dressings by exchanging counter-cation for infected full-thickness skin wounds. Mater Sci Eng C Mater Biol Appl 107: 110321.
- Kaygusuz H, Torlak E, Akın-Evingur G, Ozen I, von Klitzing R, et al. (2017) Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing. Int J Biol Macromol 105: 1161-1165.
- Singh B, Varshney L, Francis S, Rajneesh (2017) Designing sterile biocompatible moxifloxacin loaded trgacanth-PVA-alginate wound dressing by radiation crosslinking method. Wound Medicine 17: 11-17.
- Kamoun EA, Kenawy ES, Tamer TM, El-Meligy MA, Eldin MSM (2015) Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arabian Journal of Chemistry 8: 38-47.
- Yu W, Jiang YY, Sun TW, Qi C, Zhao H, et al. (2016) Design of a novel wound dressing consisting of alginate hydrogel and simvastatin-incorporated mesoporous hydroxyapatite microspheres for cutaneous wound healing. RSC Advances 6: 104375-104387.
- Ghalei S, Nourmohammadi J, Solouk A, Mirzadeh H (2018) Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf B Biointerfaces 172: 82-89.
- Boontheekul T, Kong HJ, Mooney DJ (2005) Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26: 2455-2465.
- Yadav P, Yadav H, Shah VG, Shah G, Dhaka G (2015) Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J Clin Diagn Res 9: Ze21-5.
- Gomez CG, Rinaudo M, Villar MA (2007) Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydrate Polymers 67: 296-304.
- Ulery BD, Nair LS, Laurencin CT (2011) Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys 49: 832-864.
- Chen H, Xing X, Tan H, Jia Y, Zhou T, et al. (2017) Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C Mater Biol Appl 70: 287-295.
- Xie H, Chen X, Shen X, He Y, Chen W, et al. (2018) Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int J Biol Macromol 107: 93-104.
- Rudyardjo, D.I. and S. Wijayanto, The synthesis and characterization of hydrogel chitosan-alginate with the addition of plasticizer lauric acid for wound dressing application. Journal of Physics: Conference Series, 2017. 853: p. 012042.
- Xing N, Tian F, Yang J, Li YK (2012) Characterizations of Alginate-Chitosan Hydrogel for Wound Dressing Application. Advanced Materials Research 490-495: 3124-3128.
- Mndlovu H, du Toit LC, Kumar P, Marimuthu T, Kondiah PPD, et al. (2019) Development of a fluid-absorptive alginate-chitosan bioplatform for potential application as a wound dressing. Carbohydr Polym 222: p. 114988.
- Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A (2005) Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 26: 6335-6342.
- Saarai A, Vera A, Tomas S, Petr S (2011) A comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. World Scientific and Engineering Academy and Society (WSEAS): Corfu Island, Greece, pp: 384-389.
- Saarai A, Sedlacek O, Kasparkova V, Kitano T, Saha P (2012) On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. Journal of Applied Polymer Science 126: E79-E88.
- Siqueira P, Siqueira E, de Lima AE, Siqueira G, Pinzón-Garcia AD, et al. (2019) Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 9: 78.
- Zeng Q, Han Y, Li H, Chang J (2015) Design of a thermosensitive bioglass/agarose–alginate composite hydrogel for chronic wound healing. J Mater Chem B 3: 8856-8864.
- Huang B, Liu M, Long Z, Shen Y, Zhou C (2017) Effects of halloysite nanotubes on physical properties and cytocompatibility of alginate composite hydrogels. Mater Sci Eng C Mater Biol Appl 70: 303-310.
- Lv X, Liu Y, Song S, Tong C, Shi X, et al. (2019) Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydrate Polymers 205: 312-321.
- Zhou Q, Kang H, Bielec M, Wu X, Cheng Q, et al. (2018) Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydrate Polymers 197: 292-304.
- Reakasame S, Boccaccini AR (2018) Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 19: 3-21.
- Lee SM, Park K, Kim YS, Kim HJ, Moon H, et al. (2016) Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater Res 20: 15.
- Hegge AB, Andersen T, Melvik JE, Bruzell E, Kristensen S, Tønnesen HH (2011) Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: studies on curcumin and curcuminoides XLII. J Pharm Sci 100: 174-185.
- Boateng J, Burgos-Amador R, Okeke O, Pawar H (2015) Composite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int J Biol Macromol 79: 63-71.
- Gowda D, Fredric S, Yashashwini M (2015) Wafers for wound healing. J Chem Pharm Res 7: 450-468.
- Zhang Y, Lim CT, Ramakrishna S, Huang ZM (2005) Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med 16: 933-946.
- Hu C, Gong RH, Zhou FL (2015) Electrospun Sodium Alginate/Polyethylene Oxide Fibers and Nanocoated Yarns. International Journal of Polymer Science 12: 126041.
- Shalumon KT, Anulekha KH, Nair SV, Nair SV, Chennazhi KP, et al. (2011) Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol 49: 247-254.
- Hajiali H, Summa M, Russo D, Armirotti A, Brunetti V, et al. (2016) Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. Journal of Materials Chemistry B 4: 1686-1695.
- Dahlin RL, Kasper FK, Mikos AG (2011) Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev 17: 349-364.
- Andreu V, Mendoza G, Arruebo M, Irusta S (2015) Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials (Basel) 8: 5154-5193.
- Shi M, Zhang H, Song T, Liu X, Gao Y, et al. (2019) Sustainable Dual Release of Antibiotic and Growth Factor from pH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Applied Materials & Interfaces 11: 22730-22744.
- Visscher M, Hoath SB, Conroy E, Wickett RR (2001) Effect of semipermeable membranes on skin barrier repair following tape stripping. Arch Dermatol Res 293: 491-499.
- Fernandez-Castro M, Martín-Gil B, Peña-García I, López-Vallecillo M, García-Puig ME (2017) Effectiveness of semi-permeable dressings to treat radiation-induced skin reactions. A systematic review. Eur J Cancer Care (Engl) 26.
- Weller CD, Team V, Sussman G (2020) First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front Pharmacol 11: 155.
- Roth A, Elkashif A, Selvamani V, Stucky RA, Seleem MN, et al. (2020) Wearable and Flexible Ozone Generating System for Treatment of Infected Dermal Wounds. Front Bioeng Biotechnol 8: 458.
- Zhang J, Guan M, Xie C, Luo X, Zhang Q, et al. (2014) Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev 2014: 273475.
- Broussard KC, Powers JG (2013) Wound dressings: selecting the most appropriate type. American journal of clinical dermatology 14: 449-459.
- Mian M, Beghè F, Mian E (1992) Collagen as a pharmacological approach in wound healing. Int J Tissue React 14: 1-9.
- Zhang Y, Nguyen A, Chun M, Lin Z, Ross J, et al. (2020) Ninety Days in: A Comprehensive Review of the Ongoing COVID-19 Outbreak. Health Science Journal 14: 1-13.
- Qureshi D, Nayak SK, Maji S, Anis A, Kim D, et al. (2019) Environment sensitive hydrogels for drug delivery applications. European Polymer Journal 120: p. 109220.










