Keywords
Lead neurotoxicity; Spatial learning; Memory; Neurogenesis; Doublecortin
Introduction
Despite being toxic, lead (Pb) is excessively used in industry thereby making it impossible to avoid exposure [1]. The major sources that contribute Pb to the environment in Kuwait are spray painting, metallurgical and automobile workshops and traditional cosmetics [2]. Though it has no physiological functions, yet Pb enters the body. Absorption and retention in the body depends on age, chemical environment of the respiratory and gastro-intestinal tracts and nutritional status of the individual. Generally a condition that favors calcium absorption also favors Pb absorption and retention. The total Pb content in the body does not affect its absorption in the gastrointestinal tract, as there is no feed-back mechanism for regulating its absorption [3]. After absorption, the mean half-life of Pb in blood is about 30 days and in soft tissues, such as brain, is about 40 days [4], after which majority (70-95%) of it is accumulated in bones [5].
Pb toxicity affects all organ systems. The severity of these toxic effects depends on the duration of exposure, dose and the developmental stage of the subjects. The central nervous system is affected by low doses [6,7]. It is most vulnerable during development because of high rate of mitotic activity, cellular differentiation and synaptogenesis [8,9]. Pb has the ability to substitute Ca2+, it can, therefore, concentrate in the brain [10,11]. A recent study showed that majority of infants born in Kuwait during a two-month period in 2011 had blood Pb levels above the then established reference blood Pb level of 10 μg/dL [2]. Several studies have reported poor cognitive performance with blood Pb levels as low as 3 μg/dL [12,13]. The reference blood Pb level was revised by the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) in January 2012 from 10 μg/dL to 5 μg/dL (www.cdc.gov). Blood Pb levels of > 70 μg/dL may be severely and often fatally toxic. Levels as low as 7 μg/dL in children are associated with growth retardation, impairment of cognitive and behavioral development and deficits in psychometric intelligence [14]. It has been estimated that for each 10 μg/dL increase in blood level of Pb, there is a decrease of 4-7 points in the mean IQ score [12]. Different functional aspects of the nervous system are affected at different ages [15]. Some of the adverse effects are permanent, leaving the children mentally handicapped for life. Thus, these toxic effects may have severe personal, social and economic consequences in the adult life of the individual and in the community at large.
Pb preferentially distributes in specific areas of the brain with the highest levels observed in the hippocampus and cerebral cortex, areas which are associated with learning and memory [16]. Hippocampus has been particularly shown to be affected by Pb toxicity [17]. The effect of Pb exposure on the developing nervous system has been widely reported in animal models. Pb exposure can cause structural perturbations by affecting a number of developmental processes due to effects on multiple cellular targets and cell types, including neurons and neuroglia. Such long-term phenotypic changes may involve alterations of gene expression and/or function [18]. Based on the above concerns, this study was carried out to investigate the effects of low-dose exposure to Pb during pregnancy on neurogenesis in hippocampus of young rats and spatial learning and memory.
Materials and Methods
Animals and experimental design
Wistar rats used for this study were maintained in the Animal Resources Center, Faculty of Medicine, Kuwait University. The animals were housed at constant temperature (21 ± 2°C) and relative humidity (50 ± 10%) with a 12 hour light/dark cycle (07:00 – 19:00 hours). Food and water were available all the time. The animal treatment and maintenance were according to the approved protocol of the Institutional Animal Care and Use Committee of Kuwait University. Rats were mated and after pregnancy was established (confirmed by vaginal smear), pregnant rats (n=10) were given 0.1% Pb acetate (Sigma-Aldrich, USA) dissolved in deionized water to drink from day one of pregnancy until parturition. Control animals (n=10) were given deionized water to drink throughout the study. After parturition, Pb-exposed dams were switched to deionized water. Pups born on the same day were randomly culled to 6 per dam on 5th postnatal day (PND). Male and female pups were not separated at birth, thus each litter contained both sexes. Randomly selected pups from Pb-exposed and control dams were sacrificed on PND 0, 21, 30, 40 and 50 to measure the Pb content in the hippocampus (n=6 in each group for each PND; one pup was selected from each dam). A total of 8 rats from both the control and Pb-exposed groups were used for water maze test on PND 21 and 30 (one pup was selected from each dam). After the water maze test (on PND 40 or PND 50) six randomly selected rats were perfused for doublecortin (DCX) immunostaining. Thus, in each experiment, the number of rats used represented all the dams from control and Pb-exposed groups.
Dissection of hippocampus and lead analysis
Animals were deeply anesthetized with CO2, perfused transcardially with 50 mL of cold saline and brain was removed. Hippocampus was quickly dissected in cold saline and stored at -80°C. Pb content in the hippocampal samples was measured using a Sensa Graphite Furnace (System 2000/3000) Atomic Absorption Spectrophotometer (GBC Scientific Equipment Company, USA) as described earlier 19]. Briefly, hippocampal tissue (50-100 mg) was digested by incubating overnight in 5 ml nitric acid in a fume hood. The samples were then heated at 50°C for 30 minutes and boiled at 90°C for 15 minutes to obtain a clear solution. The samples were diluted with double deionized water (Millipore) and measured in the Atomic Absorption Spectrophotometer with standard solutions containing known amount of Pb. Pb values were expressed as μg/g wet tissue weight.
Morris water maze test
The effect of Pb on spatial learning and memory was tested using the Morris water maze system [20]. Control and Pbexposed rats on PND 21 (tested from PND 21 to 27) and PND 30 (tested from PND 30 to 37) were subjected to the test. The water maze apparatus consisted of a water tank of 2.0 meters in diameter, divided into 4 virtual quadrants. A circular platform was submerged in one of the quadrant (target quadrant/platform quadrant). The rats were trained in the water maze in 11 sessions on 6 consecutive days (one session on the 1st day and two sessions on the remaining 5 days). Each session consisted of 4 trials; each trial was of 120 seconds duration. Inter-trial interval was 60 seconds. In each trial time taken to reach the hidden platform was measured and analyzed using EzVideoTM 5.70 Digital Video Tracking system (Accuscan Instruments, Inc., Columbus, OH, USA). Twenty-four hours and 10 days after the last learning session rats were subjected to memory retention test (probe test, short-term and long-term memory retention tests, respectively). Memory retention tests for PND 21-group of rats were done on PND 28 and PND 37; for PND 30-group of rat’s memory retention tests was done on PND 38 and PND 47. Each probe test session was of 30 seconds duration; distance traveled in the target quadrant was measured. Less distance traveled in the target quadrant during retention test suggested memory impairment. Rats were subjected to relearning session after long-term memory retention test on PND 38 or PND 47. This consisted of 2 sessions, each session with 4 trials. Memory retention was tested 6 hours and 24 hours after the last relearning session.
Immunostaining for DCX and neuronal quantification
Rats were perfused with 100 ml of normal saline followed by 150 ml of 4% paraformaldehyde in 100 mM phosphate buffer, pH 7.4 at 4°C. Cranium was opened and the brain was transferred to the fixative for 48 h at 4°C. Specimens were washed overnight in cold buffer, cryoprotected in 10%, 20% and 30% sucrose solution (24 hours each). To stain and quantify the number of newly generated neurons, free floating serial brain cryosections (30 μM) were processed for DCX immunostaining [21]. The primary antibody was obtained from Santa Cruz, CA, USA and all other immunostaining reagents were obtained from Vector Laboratories, CA, USA. Briefly, sections were washed in phosphate buffered saline (PBS), containing 20% methanol and 3% H2O2 to block endogenous peroxidase activity. Sections were incubated in PBS containing 5% normal horse serum and 0.01% Triton X-100 for 30 minutes. Sections were incubated with goat anti-DCX antibody (1:250) overnight at 4°C. The sections were washed and incubated for 1 hour in biotinylated horse anti-goat secondary antibody, followed by avidin-biotin complex for one hour. Diaminobenzedine was used as a chromogen. The newly generated (DCX-positive) neurons were counted using a light microscope (NIS – Elements Image Analysis System). Serial sections were collected in 24-well plates (5 sections/well). Every 10th section from each brain was used for counting DCX positive neurons. A total of 10 sections from each brain (covering the entire extent of the hippocampus) were selected for quantification of new neurons. Number of neurons was expressed as mean number/section. In each section, area of sampling and area of granule cell layer-subgranular zone (GCLSGZ)- was measured with NIS-Elements Image analysis system. Finally, absolute number of neurons in the entire dentate gyrus was calculated using the formula N = 1/ssf x 1/asf x 1/hsf x EQ– [21,22]. {ssf = sampling fraction, which was 10 in this study as every 10th section was sampled; asf = area sampling fraction, which is calculated by dividing the area sampled with the total area of the GCL-SGZ (i.e., the sum of GCL-SGZ area in every 10th section); hsf = height sampling fraction, which is calculated by dividing the height sampled (i.e., 15 μM in this study) with the section thickness at the time of analysis (i.e., 20 μM); and EQ– = the total count of particles sampled for the entire dentate gyrus}.
Statistical methods
Data were expressed as mean ± SEM/SD. One-way ANOVA followed by Bonferroni’s multiple comparisons post-test was used to compare Pb levels in the hippocampus. Student’s t-test was used to compare the performance of rats in the water maze test and DCX-positive neurons. Values of p<0.05 were considered as statistically significant.
Results
Pb level in the hippocampus
Pb levels in hippocampus are shown in Figure 1. The level of Pb in Pb-exposed rats was significantly higher on PND 0, 21, 30, 40 and 50 as compared to age matched controls. The level of Pb in hippocampus of Pb-exposed rats on PND 0 was significantly higher when compared with the Pb level in the hippocampus of Pb-exposed rats on PND 21. Similarly, the level of Pb in hippocampus of Pb-exposed rats on PND 21 was significantly higher when compared with the Pb level in the hippocampus of Pb-exposed rats on PND 30. However, no significant difference was noted in the level of Pb in hippocampus of Pb-exposed rats on PND 30 when compared with rats on PND 40. Likewise, comparison of Pb level in hippocampus of rats on PND 40 with rats on PND 50 showed no significant difference.
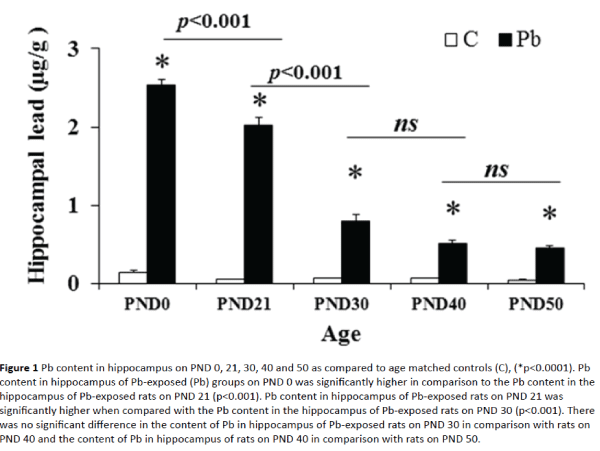
Figure 1: Pb content in hippocampus on PND 0, 21, 30, 40 and 50 as compared to age matched controls (C), (*p<0.0001). Pb content in hippocampus of Pb-exposed (Pb) groups on PND 0 was significantly higher in comparison to the Pb content in the hippocampus of Pb-exposed rats on PND 21 (p<0.001). Pb content in hippocampus of Pb-exposed rats on PND 21 was significantly higher when compared with the Pb content in the hippocampus of Pb-exposed rats on PND 30 (p<0.001). There was no significant difference in the content of Pb in hippocampus of Pb-exposed rats on PND 30 in comparison with rats on PND 40 and the content of Pb in hippocampus of rats on PND 40 in comparison with rats on PND 50.
Learning and memory performance by PND 21 group
PND 21 rats both in control and Pb-exposed groups learnt to escape on to the platform. The escape latency decreased successively from session to session and reached as low as about 35 seconds in the 4th session (35.39 ± 8.34 seconds in control group and 32.53 ± 7.88 seconds in Pb-exposed group, (Figure 2A). There was no significant difference in the escape latency between groups in all sessions, however, escape latency of session 11was significantly decreased in comparison to session 1 in both control and Pb-exposed groups (p<0.0001), suggesting proper learning ability in both groups at this age.
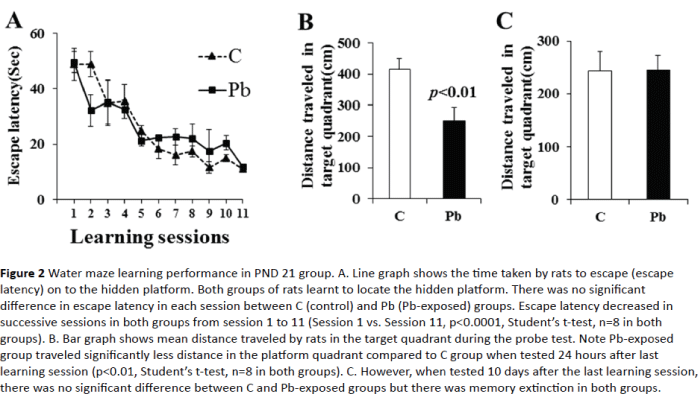
Figure 2: Water maze learning performance in PND 21 group. A. Line graph shows the time taken by rats to escape (escape latency) on to the hidden platform. Both groups of rats learnt to locate the hidden platform. There was no significant difference in escape latency in each session between C (control) and Pb (Pb-exposed) groups. Escape latency decreased in successive sessions in both groups from session 1 to 11 (Session 1 vs. Session 11, p<0.0001, Student’s t-test, n=8 in both groups). B. Bar graph shows mean distance traveled by rats in the target quadrant during the probe test. Note Pb-exposed group traveled significantly less distance in the platform quadrant compared to C group when tested 24 hours after last learning session (p<0.01, Student’s t-test, n=8 in both groups). C. However, when tested 10 days after the last learning session, there was no significant difference between C and Pb-exposed groups but there was memory extinction in both groups.
The memory retention (probe) test 24 hour after the last learning session revealed significant short-term memory deficit in Pb-exposed rats (Figure 2B). These rats entered the target quadrant after entering other quadrants, left the target quadrant and traveled significantly less distance in the target quadrant (413.44 ± 34.61cm in control vs. 250.95 ± 41.34 cm in Pb-exposed group, p=0.01). Memory retention test 10 days after the last learning session showed no significant difference between groups (Figure 2C); both control and Pb-exposed rats showed memory extinction. They explored all the quadrants equally.
Memory retention test 6 hour after the last relearning session showed no significant difference between control and Pb-exposed rats (Figure 3A). Pb-exposed rats remembered the platform quadrant very well, reached the platform quadrant quickly and traveled more than 400 cm in the target quadrant similar to the control rats (418.03 ± 28.46 cm in control and 469.83 ± 83 cm in Pb-exposed group, p>0.05). However, memory retention test 24 hours after the last relearning session revealed a significant memory deficit in the Pbexposed rats (Figure 3B). Control rats could remember the platform quadrant and reached there quickly and traveled more than 350 cm in the target quadrant as they did in probe test 6 hours after the relearning session. However, Pb-exposed rats could not remember the platform quadrant and travelled less than 225 cm in the target quadrant (364.45 ± 36.29 cm in control and 221.88 ± 21.26 cm in Pb-exposed group, p<0.01) unlike they did in probe test 6 hours after the relearning session.
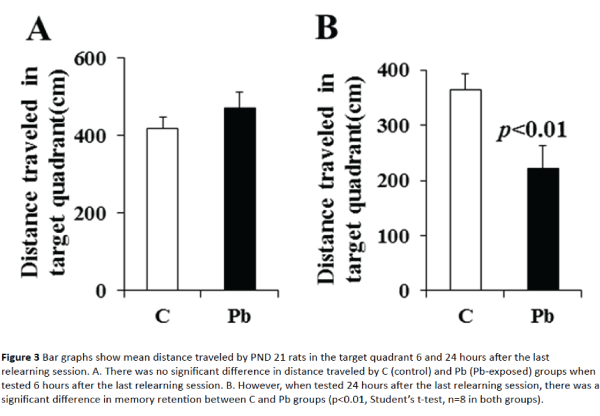
Figure 3: Bar graphs show mean distance traveled by PND 21 rats in the target quadrant 6 and 24 hours after the last relearning session. A. There was no significant difference in distance traveled by C (control) and Pb (Pb-exposed) groups when tested 6 hours after the last relearning session. B. However, when tested 24 hours after the last relearning session, there was a significant difference in memory retention between C and Pb groups (p<0.01, Student’s t-test, n=8 in both groups).
Learning and memory performance by PND 30 group
PND 30 rats in Pb-exposed group differed significantly from control rats in their learning ability (Figure 4A). Although escape latency in Pb-exposed group decreased successively from session to session, it reached less than 30 seconds in the 7th session, whereas, in control group escape latency reached less than 30 seconds as early as the 2nd session (23.68 ± 4.14 seconds in Pb-exposed group in session 7 and 28.06 ± 5.11seconds in control group in session 2) suggesting delayed learning. There was a significant difference in the escape latency between groups in sessions 1- 8, suggesting learning ability deficit in Pb-exposed group at this age (p<0.05). However, there was no difference in the learning ability of the two groups in later learning sessions (sessions 9 - 11).
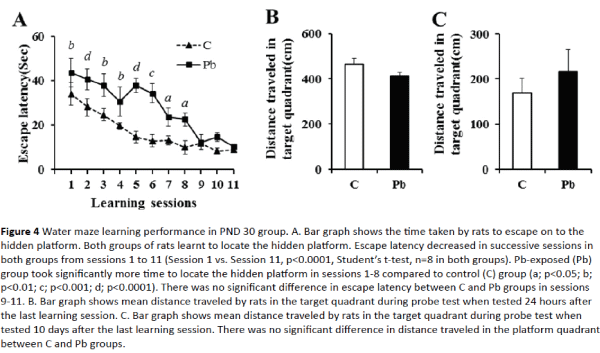
Figure 4: Water maze learning performance in PND 30 group. A. Bar graph shows the time taken by rats to escape on to the hidden platform. Both groups of rats learnt to locate the hidden platform. Escape latency decreased in successive sessions in both groups from sessions 1 to 11 (Session 1 vs. Session 11, p<0.0001, Student’s t-test, n=8 in both groups). Pb-exposed (Pb) group took significantly more time to locate the hidden platform in sessions 1-8 compared to control (C) group (a; p<0.05; b; p<0.01; c; p<0.001; d; p<0.0001). There was no significant difference in escape latency between C and Pb groups in sessions 9-11. B. Bar graph shows mean distance traveled by rats in the target quadrant during probe test when tested 24 hours after the last learning session. C. Bar graph shows mean distance traveled by rats in the target quadrant during probe test when tested 10 days after the last learning session. There was no significant difference in distance traveled in the platform quadrant between C and Pb groups.
Memory retention (probe) test 24 hours after the last learning session revealed no significant short term memory deficit in Pb-exposed rats (Figure 4B). Both groups of rats entered the target quadrant quickly and traveled significant distance in the target quadrant (467.58 ± 24.99 cm in control vs. 412.15 ± 18.24 cm in Pb-exposed group). Both control and Pb-exposed rats showed memory extinction as revealed by memory retention test 10 days after the last learning session (Figure 4C); they explored all the quadrants equally.
Memory retention test 6 hours after the last relearning session showed no significant difference between control and Pb-exposed rats (Figure 5A). Both groups of rats remembered the platform quadrant well, reached the platform quadrant quickly and traveled more than 350 cm in the target quadrant (370.67 ± 30.81cm in control and 367.67 ± 43.68 cm in Pb-exposed group). Moreover, memory retention test 24 hours after the relearning session (Figure 5B) did not show memory deficit. The rats remembered the platform quadrant. There was no significant difference in distance travelled by control and Pb-exposed rats (272.45 ± 31.89 cm in control vs. 284.80 ± 23.18 cm in Pb-exposed rats; p > 0.05). The results of water maze tests are summarized in Table 1.
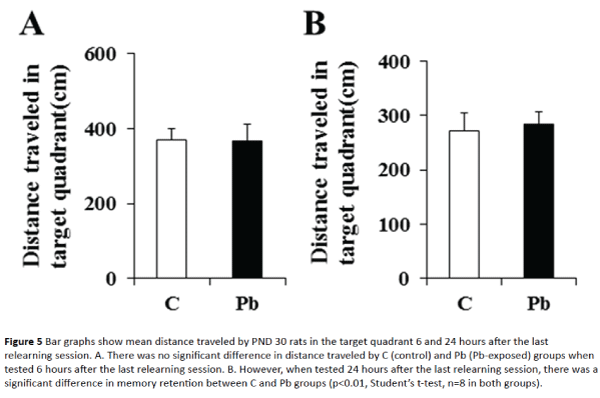
Figure 5: Bar graphs show mean distance traveled by PND 30 rats in the target quadrant 6 and 24 hours after the last relearning session. A. There was no significant difference in distance traveled by C (control) and Pb (Pb-exposed) groups when tested 6 hours after the last relearning session. B. However, when tested 24 hours after the last relearning session, there was a significant difference in memory retention between C and Pb groups (p<0.01, Student’s t-test, n=8 in both groups).
| |
Pb-exposed rats (Compared to controls) |
Pb-exposed PND 30 group vs. PND 21 group |
| |
PND 21 group |
PND 30 group |
| Learning ability |
No deficit(PND 21-26) |
Delayed learning(PND 30-36) |
Delayed |
| Short term memory |
Memory retentiondeficit (on PND 27) |
Good memory retention (on PND 37) |
Good |
| Long term memory |
Memory extinction(on PND 37) |
Memory extinction(on PND 47) |
No difference |
| Short term memory6 h after relearning |
Good memory retention (on PND 38) |
Good memory retention (on PND 48) |
Good |
| Short term memory 24 h after relearning |
Memory retentiondeficit (on PND 39) |
Good memory retention (on PND 49) |
Good |
Pb content in hippocampus was high throughout the experimental period in Pb-exposed
PND 21 and 30 rats compared to age matched controls.
Learning and memory was better in PND 30 rats, even though hippocampal neurogenesisdecreased in both groups compared to age matched controls. |
Table 1: Performance of rats in water maze.
Hippocampal neurogenesis
Figures 6-7 show significantly decreased adult neurogenesis in the dentate gyrus at both PND 40 (PND 21 group) and PND 50 (PND 30 group) in Pb-exposed rats compared to control rats. In control PND 40 rats, dentate gyrus showed densely packed DCX-positive neurons in the crest (CR), suprapyramidal (SPB) and infrapyramidal blade (IPB) regions (Figure 6A). These neurons were of mature nature as majority of new neurons were vertically oriented and exhibited extensive dendrites that extended to the outer molecular layer of the dentate gyrus in all the subregions (Figure 6B-6D). In Pb-exposed PND 40 rats, dentate gyrus exhibited decreased number of DCX-positive neurons in the crest, suprapyramidal and infrapyramidal blade regions (Figure 6E). Majority of these new neurons were of immature nature as they were horizontally oriented, located in the dentate hilus with shorter dendrites confined to the inner molecular layer of the dentate gyrus in all the subregions (Figures 6F-6H).
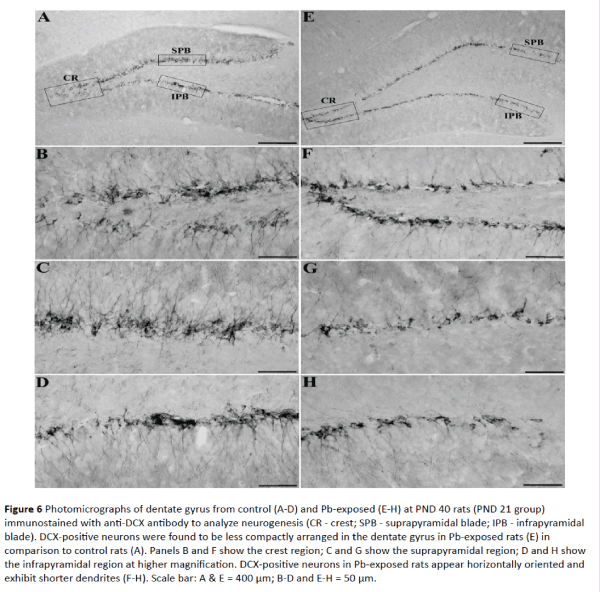
Figure 6: Photomicrographs of dentate gyrus from control (A-D) and Pb-exposed (E-H) at PND 40 rats (PND 21 group) immunostained with anti-DCX antibody to analyze neurogenesis (CR - crest; SPB - suprapyramidal blade; IPB - infrapyramidal blade). DCX-positive neurons were found to be less compactly arranged in the dentate gyrus in Pb-exposed rats (E) in comparison to control rats (A). Panels B and F show the crest region; C and G show the suprapyramidal region; D and H show the infrapyramidal region at higher magnification. DCX-positive neurons in Pb-exposed rats appear horizontally oriented and exhibit shorter dendrites (F-H). Scale bar: A & E = 400 μm; B-D and E-H = 50 μm.
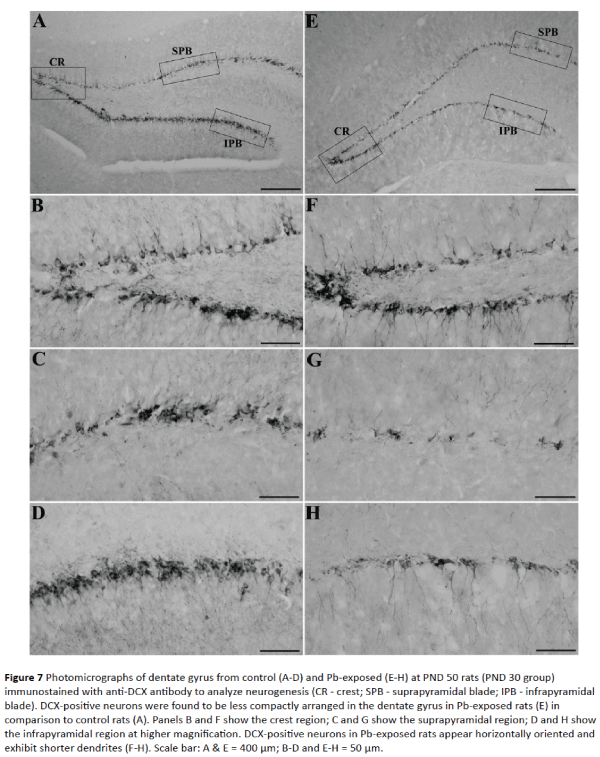
Figure 7: Photomicrographs of dentate gyrus from control (A-D) and Pb-exposed (E-H) at PND 50 rats (PND 30 group) immunostained with anti-DCX antibody to analyze neurogenesis (CR - crest; SPB - suprapyramidal blade; IPB - infrapyramidal blade). DCX-positive neurons were found to be less compactly arranged in the dentate gyrus in Pb-exposed rats (E) in comparison to control rats (A). Panels B and F show the crest region; C and G show the suprapyramidal region; D and H show the infrapyramidal region at higher magnification. DCX-positive neurons in Pb-exposed rats appear horizontally oriented and exhibit shorter dendrites (F-H). Scale bar: A & E = 400 μm; B-D and E-H = 50 μm.
In control PND 50 rats, dentate gyrus showed densely packed DCX-positive neurons in the crest, suprapyramidal and infrapyramidal blade regions similar to that seen in PND 40 rats (Figure 7A). These neurons were of mature nature as majority of new neurons were vertically oriented and with extensive dendrites that extended to the outer molecular layer of the dentate gyrus in all the sub regions (Figures 7B-7D). In Pb-exposed PND 50 rats, dentate gyrus showed fewer DCX-JOURNAL positive neurons in the crest, suprapyramidal and infrapyramidal blade regions (Figure 7E). Majority of these new neurons were of immature nature as they were horizontally oriented, located in the dentate hilus and exhibited shorter dendrites confined to the inner molecular layer of the dentate gyrus in all subregions (Figures 7F-7H).
There was a significant decrease in the number of new (DCX-positive) neurons in the entire dentate gyrus of Pb-exposed rats at PND 40 and 50 as compared to age-matched control rats (Figure 8). At PND 40 and PND 50 the total number of DCX-positive neurons in the entire dentate gyrus was 55.92% and 53.56% less in Pb-exposed group in comparison to control group. These values were statistically significant (p<0.0001).
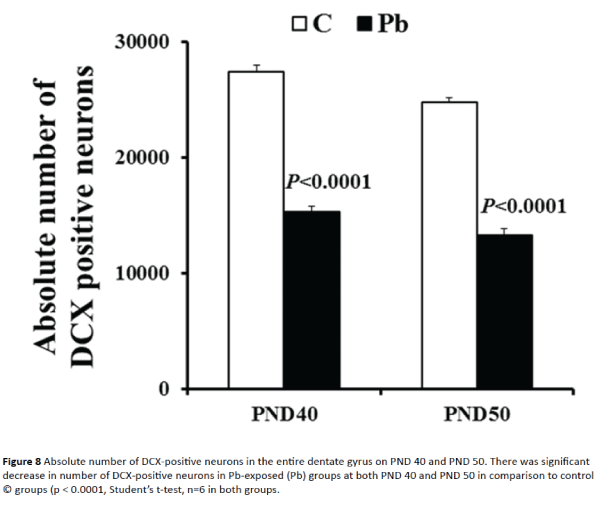
Figure 8: Absolute number of DCX-positive neurons in the entire dentate gyrus on PND 40 and PND 50. There was significant decrease in number of DCX-positive neurons in Pb-exposed (Pb) groups at both PND 40 and PND 50 in comparison to control © groups (p < 0.0001, Student’s t-test, n=6 in both groups.
Discussion
We report here that low-dose Pb exposure during pregnancy causes cognitive impairment in rats. Our results show that in utero exposure to Pb caused significant increase in the Pb levels in the hippocampus. This resulted in deficits in spatial learning and memory ability at PND 40 but not at PND 50. However, neurogenesis decreased at both time points. This disparity in spatial learning and memory may possibly be due to some unknown compensatory mechanism in PND 50 rats.
Pb and neurotoxicity
Despite innumerable studies over last several decades there is consensus only on one issue that human exposure to Pb raises the level of Pb in blood and subsequently in the brain resulting in functional deficits [23]. Our data show significantly high levels of Pb in the hippocampus on PND 0 (2.5 μg/g) that came down to about 0.5 μg/g on PND 50. This high level of Pb in the hippocampus during the period of growth and maturation of neurons could cause morphological and physiological alterations and could lead to learning and memory deficits. Studies on murine and rodent models have shown the deficits in spatial memory and learning in young and mature animals but there is no agreement on the mechanism or mechanisms of Pb-induced neurotoxicity. It was suggested that Pb may act as a chemical stressor that could cause breakdown of homeostatic cellular mechanisms. Such cellular mechanisms may be expressed as structural and physiological changes thereby modulating emotional response, learning and memory [7]. Exposure to Pb is known to increase the threshold and decrease the magnitude for long-term potentiation in hippocampus, affect adult neurogenesis, cause perturbations in synaptic plasticity and cause cognitive deficits [24]. Chronic exposure to Pb is reported to alter the Bax/Bcl-2 ratio in rat hippocampus [25] and Pb is also known to affect the PI3K/AKT pathway causing death in cultured hippocampal neurons [26]. Pb was also shown to over-activate the hippocampal serine/threonine protein phosphatases PP1 and PP2A and cause learning and memory deficits in young rats [19] and also induce tau phosphorylation in the brain of young rats [27]. It must be noted that as the brain grows with age the amount of Pb/g tissue decreases. Although we did not quantify the blood Pb level in our animals, but it is reported that after the animals are provided Pb-free water the level of Pb in blood and brain shows a decline [30] [19] and it gets accumulated in bones [28].
Spatial learning and memory
We observed that though the Pb-exposed rats were slow learners as compared to the control rats but with repeated learning sessions they were able to locate the target quadrant. The Pb-exposed PND 40 (PND 21group) rats matched the learning ability of the control group in all 11 learning sessions. The Pb-exposed PND 50 (PND 30 group) rats differed from the control group in learning sessions 1-8. The results of the probe test for the short-term memory showed significant difference between Pb-exposed and control PND 40 rats, but the probe test results between the PND 50 Pb-exposed and the control rats showed no difference. In both PND 21 and PND 30 rats there was no difference in the long-term memory when compared with the control rats. However, the results of an earlier study, similar to ours where dams were subjected to Pb-exposure through gestational period only, showed quite different results. These authors reported long-term learning/ memory deficits in young adults [29]. In a study where the dams were exposed to Pb prior to mating, during pregnancy and lactation, results showed age-dependent impairment of performance in the water maze [30]. In a later study, when low-level Pb-exposure lasted from gestational day 16 to PND 21, there was a negative effect on neurogenesis in the hippocampus, but there was no significant impact on spatial learning [31]. In a comparable study on human subjects, it was noted that the Pb-exposure during the first trimester was predictive of adverse neurodevelopment later in life as indicated by the Medical Development Index scores [32]. In yet another report where the authors studied the effects of perinatal (gestation to weaning) exposure to Pb, the results were quite interesting. The authors concluded that the environmental manipulation was a potential risk modifier of developmental Pb exposure. Furthermore, the authors stated that sex and amount of Pb exposure affected the functional influence of Pb on brain [33]. Thus, results of human and animal studies have reported conflicting results in terms of short- and long-term memory. These differences could be attributed to alterations in the dose and duration of Pb exposure, route of administration, developmental stage (gestation, lactation or weaning), and the PND at which the test was conducted.
Pb and neurogenesis
Learning and memory deficits have been associated with decreased neurogenesis. Since hippocampus is associated with learning and memory, studies have reported the effect of Pb on hippocampal neurogenesis [31,34]. However, both these studies used bromodeoxyuridine (BrdU), a marker for DNA sysnthesis that labels proliferating cells and their progeny. Although a marker for new cells, BrdU is not specific for new neurons. We used DCX, a microtubule-associated protein that is expressed specifically in neuronal precursors in the central nervous system [35]. Our results show significant reduction in neurogenesis in the dentate gyrus of Pb-exposed rats (PND 21 and PND 30 groups). Not only that the production of new neurons was affected in our animals, the alignment of these neurons was affected as well. The apical dendrites of these new neurons were horizontally oriented. Such quantitative and morphological variations would not only result in abnormal neuronal circuitry but it would also affect synaptogenesis. The effects of Pb exposure on hippocampal ultrastructure [36] and on synaptogenesis are known [19,34,37]. In case there is reduction and or alteration in neuronal circuitry of the hippocampus, it would result in learning and memory deficits. Several studies have reported the effects of Pb on alterations in the expression of receptor proteins and or rerelease of neurotransmitters and learning and memory deficits (cholinotoxicity [38]; glutamate and GABA release [39,40]; mGluR1 and NMDA receptors [41,42].
In spite of decreased number of new neurons and morphological alterations, PND 30 rats exhibited better memory retention in comparison to PND 21 rats. Thus it is tempting to speculate that this may be due to the relative decrease in the level of Pb/g wet tissue weight in the hippocampus of older rats or due to some other yet unknown mechanism. Future plan is to carry out long term study on rats exposed to Pb during gestation and determine the level of Pb in whole brain, hippocampus and bone and carry out water maze test at the age of 24 months.
Our data support the notion that exposure to Pb during gestation significantly affects neurogenesis in the dentate gyrus and alters the orientation of new neurons. The altered organization of neurons affects the neuronal circuitry and synaptogenesis and may result in modifications in the expression of various receptor proteins and possibly release of neurotransmitters. These morphological changes are likely to dictate the physiological alterations that result in deficits in spatial learning and memory.
Acknowledgements
This work was supported by the Department of Anatomy. The authors acknowledge the excellent technical assistance provided by Dr. S. Jacob, Ms. S. Joe and Ms. S. Sivanandan and the support of the Animal Resources Center and the Research Core Facility (SRUL 02/13) of the Kuwait University Health Sciences Center.
9282
References
- Chen L, Yang X, Jiao H, Zhao B (2002) Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. ToxicolSci 69: 149-156.
- Rahman A, Al-Rashidi HAG, Khan AR (2012) Association of maternal blood lead level during pregnancy with child blood lead level and pregnancy outcome in Kuwait. Ecol Food Nutr 51: 40-57.
- De Michele SJ (1984) Nutrition of lead. Comp BiochemPhysiol A 78: 401-408.
- Rabinowitz MB (1990) Toxicokinetics of bone lead. Environ. Health Perspect 91: 33-37.
- Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H (2012) Alzheimer’s disease and environmental exposure to lead: The epidemiologic evidence and potential role of epigenetics. Curr. Alzheimer Res 9: 563-573.
- Borja-Aburto VH, Hertz-Picciotto I, Lopez MR, Farias P, Rios C, Blanco J (1999) Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol 150: 590-597.
- Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Rev 27: 168-176.
- Hsiang J, Diaz E (2011) Lead and developmental neurotoxicity of the central nervous system. Curr Neurobiol 2: 35-42.
- Lockitch G (1993) Perspectives on lead toxicity. Clin. Biochem 26: 371-381.
- Garza A, Vega R, Soto E (2006) Cellular mechanisms of lead neurotoxicity. Med SciMonit 12: 57-65.
- Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: A review. Rev Environ Health 24: 15-45.
- Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 µg/dL. N Engl J Med 348: 1517-1526.
- Chiodo LM, Jacobson SW, Jacobson JL (2004) Neurodevelopmental effects of postnatal lead exposure at very low levels. NeurotoxicolTeratol 26: 359-371.
- Selvin-Testa A, Loidi CF, Lopez EM, Capani F, Lopez-Costa JJ, et al. (1995) Prolonged lead exposure modifies astrocytes cytoskeletal protein in the rat brain. Neurotoxicol 16: 389-401.
- Lefauconnier JM, Bernard G, Mellerio F, Sebille A, Cesarini E (1983) Lead distribution in the nervous system of 8-month-old rats intoxicated since birth by lead. Experientia 39: 1030-1031.
- Stoltenburg-Didinger G (1994) Neuropathology of the hippocampus and its susceptibility to neurotoxic insult. Neurotoxicol 15: 445-450.
- Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH (2003) Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J DevNeurosci 21: 1-12.
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Al-Shemary T (2012) Over activation of hippocampal serine/threonine protein phosphatases PP1 and PP2A is involved in lead-induced deficits in learning and memory in young rats. Neurotoxicol 33: 370-383.
- Hattiangady B, Kuruba R, Shetty AK (2011) Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis 2: 1-17.
- Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19: 234–246.
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ (2001) Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc 204: 232–246.
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, et al. (2007) Blood lead levels and specific attention effects in young children. NeurotoxicolTeratol 29: 538-546.
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, et al. (2007) New and evolving concepts in the neurotoxicology of lead. ToxicolApplPharmacol 225: 1-27.
- Sharifi AM, Mousavi SH (2010) Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cell MolNeurobiol 30: 769-774.
- Li C, Xing T, Tang M, Yong W, Yan D, et al. (2008) Involvement of cyclin D1/CDK4 and pRb mediated by PI3K/AKT pathway activation in Pb2+-induced neuronal death in culture hippocampal neurons. ToxicolApplPharmacol 229: 351-361.
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Attur S (2012) Early postnatal lead exposure induces tau phosphorylation in the brain of young rats. ActaBiolHungarica 63: 411-425.
- Hu H, Rabinowitz M, Smith D (1998) Bone lead as biological marker in epidemiological studies of chronic toxicity: Conceptual paradigm. Environ. Health Perspect 106: 1-8.
- Yang Y, Ma Y, Ni L, Zhao S, Li L, et al. (2003) Lead exposure through gestation-only caused long-term learning/memory deficits in young adult offspring. ExpNeurol 184:489-495.
- Jett DA, Kuhlmann AC, Farmer SJ, Guilate TR (1997) Age-dependent effects of developmental lead exposure on performance in the Morris water maze. PharmacolBiochemBehav 57: 271-279.
- Gilbert ME, Kelly ME, Samsam TE, Goodman JH (2005) Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. ToxicolSci 86: 365-374.
- Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, et al. (2006) Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ. Health Perspect 114; 1730-1735.
- Anderson DW, Pothakos K, Schneider JS (2012) Sex and rearing condition modify the effects of perinatal lead exposure on learning and memory. Neurotoxicol 3:985-995.
- Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neurosci 145: 1037-1047.
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, et al. (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21: 1-14.
- Xu J, Yan HC, Yang B, Tong LS, Zou YZ, et al. (2009) Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J Neg Res Biomed 8: 5.
- Han XJ, Xiao YM, Ai BM, Hu XX, Wei Q, et al. (2014) Effects of organic selenium on lead-induced impairments of spatial learning and memory as synaptic structural plasticity. Biol Pharm Bull 37: 466-474.
- Bourjeily N, Suszkiw JB (1997) Developmental cholinotoxicity of lead: loss of septal cholinergic neuron and long-term changes in cholinergic innervation of the hippocampus in perinatally lead-exposed rats. Brain Res 771: 319-328.
- Lasley SM, Gilbert ME (2002) Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. ToxicolSci 66: 139-147.
- Lasley SM, Green MC, Gilbert ME (1999) Influence of exposure period on in vivo hippocampal glutamate and GABA release in rats chronically exposed to lead. Neurotoxicol 20: 619-629.
- Wang XM, Liu WJ, Zhang R, Zhou YK (2012) Effects of exposure to low-level lead on spatial learning and memory and the expression of mGluR1, NMDA receptor in different developmental stages of rats. Toxicol Indus Health 29: 686-696.
- Yu H, Li T, Cui Y, Liao Y, Wang G, et al. (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicol 325: 189-199.













