Keywords
|
| Gastrointestinal cancer, Metronomic chemotherapy, Capecitabine, Response, Quality of life |
Introduction
|
|
Gastrointestinal cancer
|
| Worldwide, cancer is a major public health-care problem. Cancer accounts for 25% of all deaths, thus ranked second as leading cause of death, after ischemic heart disease. In 2010, there were 1.7 million deaths and 3.2 million new cases estimated each year in Europe and due to the aging of the European population, these numbers are expected to increase [1]. Gastrointestinal cancer (GI-cancer) is a common cancer form with colorectal cancer (CRC) as the second leading cause for cancer mortality [1]. Globally, CRC is the third most common cancer disease among both males and females [2]. In addition, CRC stands for approximately 50% of all GI-cancer cases [3]. |
| GI-cancer has for many years been considered a surgical disease. The possibility of curative surgical treatment mainly depends on the size of the tumor(s) and the presence of metastases. Unfortunately, it is common that the cancer already has spread to distant sites at the time of diagnosis. A metastatic disease is often a chronic nature and, therefore, rarely cured by surgery. When cure is not possible, chemotherapy can get the disease manifestations to decrease or remain stable, thus may delay the time to disease progression, relief symptoms and improve quality of life (QoL). Within the palliative setting, the main goals are to normalize life and prolong survival without reducing QoL more than necessary [4]. |
| Unfortunately, conventional chemotherapy is associated with a lot of adverse effects that in many cases interfere with QoL. Vomiting, nausea, hair loss and neurotoxicity are some of the common side effects that the patients experience most bothersome [3]. Lowdose continuous anti-cancer treatment, also called metronomic chemotherapy is a relatively new concept of treating cancer, which is emerging as a promising alternative to the traditional regimens. This way of drug administration may offer important advantages such as higher tolerability and lower probability of drug resistance [5]. Metronomic chemotherapy has most of all been reported for breast cancer while its effect in GI-cancer is far less explored [6,7]. Although, a continuous low-dose approach is not commonly used for GI-cancer, there have been some earlier reports with promising results. Two case reports from 2007 and 2012 demonstrated successful treatments with metronomic capecitabine in patients with metastatic esophageal cancer and CRC [8,9]. In 2013, a pilot study which included 35 patients with metastatic GI-cancer was published [6]. In that study, the patients were treated with continuous low-dose capecitabine and for 6 of the 35 patients, palliation periods for more than two years were observed. |
|
Metronomic chemotherapy
|
| In order to improve cancer treatment, it is essential to explore new drug targets and treatment strategies. Within classical chemotherapy, drug resistance and toxic adverse events are two common issues [5]. According to the conventional chemotherapeutic regimens, cytostatic drugs are mainly used in combinations, given at doses near or even at the maximum tolerated dose (MTD). In order to allow the patient to recover from the treatment-related side effects, the drugs are administered in cycles (e.g. every third week) [10]. Initially, this traditional way of drug administration often results in tumor regression or stabilization, but in many cases, the response is short-lived. Nevertheless, conventional treatment is also associated with a reduced QoL [11]. In general, the concept of MTD-based chemotherapy is more effective for primary tumors than against metastasis. For a metastatic disease, conventional chemotherapy regardless if several cytotoxic drugs are combined, mostly only attain palliative effects [12]. |
| In 2000, the term metronomic chemotherapy was coined by Hanahan et al. [11]. Metronomic chemotherapy refers to continuous drug administration of chemotherapeutic drugs (e.g. daily), at low doses, without longer drug-free intervals [13]. In contrast to conventional chemotherapy where rise and fall of the plasma concentration are the features, metronomic treatment leads to a sustained plasma concentration of the drug, thereby offering a lower probability for adverse events to occur [10]. Most clinical trials have demonstrated that high-grade toxicity is rare or even not found [14]. Moreover, a continuous low-dose approach may also be more cost-effective since it offers a higher preference for inexpensive oral drugs and fever expenses caused by adverse effects [15]. |
| A consideration regarding metronomic chemotherapy is that many important aspects of the treatment are unresolved or empirical. These include the choice of chemotherapeutic agent, the optimal dose, dosing interval and patient selection [7,16,17]. In clinical practice, metronomic chemotherapy is emerging as an attractive experimental treatment option for the elderly and fragile patients who may not be fit for conventional chemotherapy, but still are motivated for treatment [16]. Metronomic chemotherapy might also be suitable within the palliative setting when the goal is to prevent disease progression without reducing QoL to a higher extent [6]. |
|
Metronomic chemotherapy - mechanisms of action
|
| Within classical chemotherapy, cytotoxic drugs have mainly been designed to act on the cancer cell itself [18]. The goal is to kill as many tumor cells as possible and thereby hopefully treat cancer [5]. Classic cytostatic drugs often act as mitotic inhibitors, alkylators, enzyme inhibitors or false metabolites in order to cause deoxyribonucleic acid (DNA) damage or inhibit mitosis [19]. In contrast to conventional chemotherapy, metronomic chemotherapy is believed to act by different mechanisms [14]. The aim of metronomic anti-cancer treatment is to reach tumor control by primary target tumor angiogenesis [5]. Angiogenesis is the process in which new blood vessels are formed. Over the past decades, tumor angiogenesis has been established to have a very important role in the control of tumor and metastatic growth [20]. The anti-angiogenic effect is thought to be mediated by killing the bone-marrow-derived endothelial progenitor cells or by either killing or inhibit the endothelial cells in the vasculature of the tumor [7,8,14]. In vivo, metronomic chemotherapy has also shown to increase the levels of thrombospondin-1, an endogenous inhibitor of angiogenesis [5]. Since the drugs are administered continuously without longer drug-free intervals, the endothelial cells do not get the chance to recover, and as a result, the anti-angiogenic effect remains sustained [14]. |
| Besides the anti-angiogenic properties of low-dose continuous chemotherapy, additional mechanisms have been revealed. These include stimulation of the immune system and induction of tumor dormancy (7). The immune system stimulation is believed to be mediated by inhibition of regulatory T-cells (Tregs) and activation of dendritic cells. Inhibition of Tregs lead to activation of tumor un-specific and tumor specific effector cells while activation of dendritic cells is thought to enhance the immunestimulatory effect [14,21-23]. Tumor dormancy may be induced through three different methods. These include immunesurveillance, suppressing angiogenesis and by causing apoptosis of cancer cells [10]. It has also been shown that metronomic chemotherapy might improve the effect of targeting drugs such as the monoclonal antibody bevacizumab [8,14]. |
|
Capecitabine (Xeloda)
|
| The efficacy of 5-Fluorouracil (5-FU) was discovered in the fifties. Since then, it has been a very important and commonly used drug for treatment of GI-cancer [6]. For treatment of colorectal cancer, 5-FU has been used for more than 40 years [24]. 5-FU is administered intravenously (I.V), which requires insertion of a tedious peripherally central catheter (PCC) [25]. New treatment strategies have been developed in order to increase the anticancer activity, but there are still factors that limit its clinical use. These include drug resistance and controversies regarding the optimal administration of the drug [6,26]. |
| The development of an oral fluoropyrimidine derivative named capecitabine (Xeloda) has opened up new possibilities regarding this question [6,27]. Xeloda is a prodrug, which is administered to the patient in a tablet formulation [28]. By using oral administration it is easier to maintain a desirable blood concentration of the drug and since placement of a PCC is not necessary, catheter-related complications are avoided [25]. After absorption from the gastrointestinal tract, capecitabine is enzymatically converted to 5-FU. The conversion to 5-FU occurs in several steps, where the enzyme responsible for the final step is called thymidine phosphorylase [29]. Since thymidine phosphorylase is located at much higher concentrations in tumor tissue than in normal tissues, the active form of the drug is mainly present at the tumor site [30]. In the tumor tissue, 5-FU is converted to fluorodeoxyuridine monophosphate and fluorouridine triphosphate. Both these metabolites cause cell injury through two mechanisms. These include incorporation into RNA as a false nucleotide and inhibition of the enzyme thymidylate synthase, which is responsible for the rate-limiting step in the thymidine synthesis [24]. Since thymidine is essential for DNA-synthesis, cellular death ensues. The incorporation of a false nucleotide into RNA leads to inhibition of DNA-synthesis followed by apoptosis [28]. |
| Capecitabine has in preclinical xenograft models possessed activity in both 5-FU-resistant and 5-FU-sensitive cancers. Furthermore, low-dose continuous capecitabine has also shown to be safe and be of antitumoral benefit in hepatocellular carcinoma. In a clinical trial including 59 previously untreated patients with advanced hepatocellular carcinoma, metronomic Xeloda achieved an overall survival and a progression free-interval of 14 and 6 months respectively [31]. In a pilot study which included 35 patients with stage IV GI-cancer treated with metronomic capecitabine, both regression and stable diseases were observed [6]. In this study, the efficacy of metronomic Xeloda will be further explored in an extended patient material with metastatic GI-cancer. |
|
Aims of the study
|
| The aim of this study was to explore the efficacy of metronomic Xeloda in a patient material with metastatic GI-cancer. The patients included were for some reason not considered suitable for evidence-based medicine. Another goal was to investigate the safety and tolerability during treatment. |
Materials and methods
|
|
Study design and patient material
|
| In this study, 87 patients with stage IV GI-cancer were included. The patients were recruited from a city in Sweden and were diagnosed with a primary metastatic or recurrent disease between 2007 and 2015. The patients started treatment with low-dose Xeloda (500 mg x 2) during the period 2009-2015 and were retrospectively followed-up 2015. Many of the patients had been treated with classical chemotherapy before treatment with metronomic Xeloda. Patients that were given metronomic Xeloda as the first line of treatment were those of high age (≥75) or with medical contraindications that were not considered fit for conventional chemotherapy. Other indications for treatment were experimental treatment or severe toxicity to prior standard chemotherapy. Experimental treatment was considered when all other evidence-based regimens already had been tested and the patient was given metronomic Xeloda as the third, fourth or fifth line of treatment. |
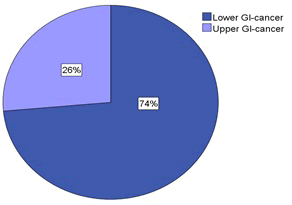
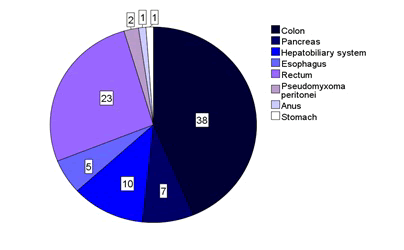
| Of the 87 patients, many were more than 70 years of age, ranging from 28 to 87 years. The patients were divided into two groups as shown in Figure 1: one group with lower GI-cancer (n = 64) and one group with upper GI-cancer (n = 23). The malignancies representing the lower GI-cancer were cancer of the colon (n = 38), rectum (n = 23), anus (n = 1) and pseudomyxoma peritonei (n = 2). The group with upper GI-cancer included cancer of the esophagus (n = 5), pancreas (n = 7), stomach (n = 1) and the hepatobiliary system (n = 10). Figure 2 shows the number of patients for each of the malignancies that were included in this study. |
| Patients were followed-up every eight week by radiological evaluation and every fourth week by clinical examination, including laboratory results. In order to assess the best response, patients had to have been under treatment for at least two months. The best response of the treatment was defined as the best response measured from the start of the treatment until disease progression. The first radiological evaluation occurred approximately two months after initiation of treatment. Assessment of tumor response was performed according to standard RECIST criteria [32]. In general, the patients were radiologically re-evaluated every 6-8 week until disease progression. For a very small proportion of the patients, the best response of the treatment was not evaluable. The primary reason to this was that there was no x-ray result available. |
|
Patient data
|
| Clinical data were obtained from an electronic health care journal system called COSMIC and from paper journals. The data were transferred into a database, which was created in Microsoft Excel. Variables recorded were age at diagnosis, date of diagnosis, date of recurrence, site of primary tumor, side effects, QoL, line of treatment, date of death, cause of death (cancer-related or other), treatment duration, indication for treatment, best response of the treatment and last date of follow-up for survivals. Other cytostatic drugs that were used in combination with metronomic Xeloda were also noted. When a patient had more than one relapse and was radically operated, the treatment lines were counted from the last recurrence. The dates of diagnosis and relapse were based on the pathological-anatomical diagnosis (PAD). In cases where no PAD was available, the dates were based on the radiological and clinical picture of the patient. Side effects were classified according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. |
|
End points
|
| The primary endpoints were to investigate the two-year overall survival (OS) for the cohort and the best response of the treatment, which was classified as stable disease, regress or progress of disease. OS was defined as the time from diagnosis to date of death (cancer-related or other causes) or date of last follow-up for patients who were alive at the time of analysis. The two-year OS was analysed within the cohort according to following parameters: age, gender, tumor position and treatment line. Other endpoints were treatment duration, treatment indications, QoL, adverse effects, but also to study whether the patients were treated with other drugs in combination with Xeloda. Quality of life and adverse effects were analyzed during treatment with Xeloda. The side effects included in this study were: diarrhea, skin rashes, neuropathies, convulsions, hand-foot syndrome, dry mucous membranes, leg swelling and redness. Treatment indications were classified as high age (>75), toxicity or experimental treatment, which was considered when all other conventional chemotherapeutic regimens already had been explored. |
|
Ethics
|
| With respect to the ethical aspects, personal data were made anonymous by giving each patient an ID-number. All patient data were safely stored throughout the whole study. The study was approved by the institutional review board of Linköping University Hospital. |
|
Statistical analysis
|
| For the patient material, a survival analysis was made using the Kaplan-Meier method. Analysis of OS within the cohort was tested for significance with the non-parametric log-rank test. P-values less than 0.05 were deemed as statistically significant. Statistical analysis was performed using the software program, IBM SPSS Statistics for Windows, version 23.0. Tables and calculations were done in Microsoft Excel. |
Results
|
|
Patient characteristics
|
| Of the 87 included patients, 57% were men (n = 50) and 43% women (n = 37). The average age for the whole cohort was 69 years. Five patients were younger than 50 years and as much as 41 patients were older than 70 years. Lower GI-cancer was the most common cancer form among both males and females. For patients with upper GI-cancer, the average age was slightly higher (70 years) than for patients with lower GI-cancer (68 years). Twenty-one percent of the patients (n = 18) were treated with metronomic Xeloda because of their high age (≥ 75) and 26% of the patients (n = 23) due to toxicity of prior treatments. The most common indication for treatment was experimental treatment, which involved 53% of the cohort (n = 46). Sixty-nine percent of the patients (n = 60) had been treated with conventional chemotherapy before treatment with metronomic Xeloda. Moreover, 31% of the patients (n = 27) were given metronomic Xeloda as the first line of treatment. Demographic and treatmentrelated variables are summarized in Table 1. |
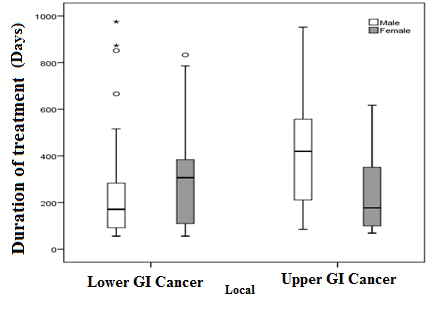
| As shown in Figure 3, treatment duration was longer for the group of patients with upper GI-cancer with a median duration of 177 and 420 days for females and males respectively. Within the group of patients who had lower GI-cancer, females had a longer median duration (307 days) than males (172 days). A few patients (n = 5) had an exceptional long treatment duration of more than 800 days. At the end of the study, 15% (n = 13) of the patients were still under treatment with metronomic Xeloda. Many patients were treated with other cytostatic drugs in combination with low-dose Xeloda. Forty-seven percent (n = 41) of the patients had combination therapy and 53% (n = 46) had monotherapy with metronomic Xeloda. The most frequent drug used in combination with Xeloda was Avastin (n = 32). Other drugs used were Vectibix (n = 7), Campto (n = 3), Erbitux (n = 2), Eloxatin (n = 2), Tamoxifen (n = 1), Tarceva (n = 1) and Sendoxan (n = 1). For some patients, more than one drug combination was used. |
|
Best response
|
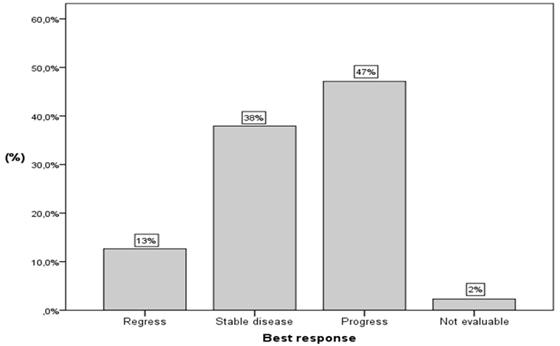
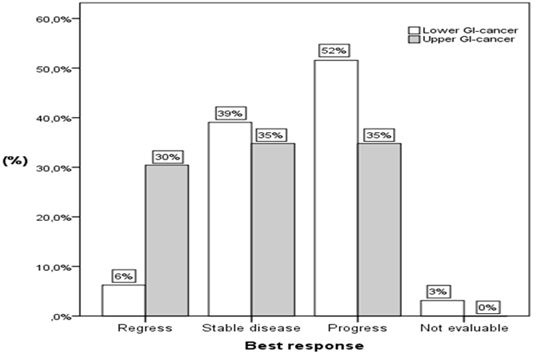
| Overall, many patients responded to the treatment and had regress or stable disease (Figure 4A). Still, progressive disease was more common. Of the whole cohort, 13% (n = 11) showed regress, 38% (n= 33) stable disease and 47% (n = 41) had progress of disease. The best response in each of the two groups (upper and lower GI-cancer) is shown in Figure 4B. Regress of the disease was observed in 30% (n = 7) of the patients with upper GI-cancer and in 6% (n = 4) of the patients with lower GI-cancer. Progressive disease was more common for lower GI-cancer. Within that group, 52% (n = 33) of the patients showed progress while 35% (n = 8) of the patients in the other group had progression as well. Stable disease was observed in 39% (n = 25) and 35% (n = 8) of the patients with lower and upper GI-cancer respectively. For two patients with lower GI-cancer, the best response was not evaluable. |
|
Survival analysis
|
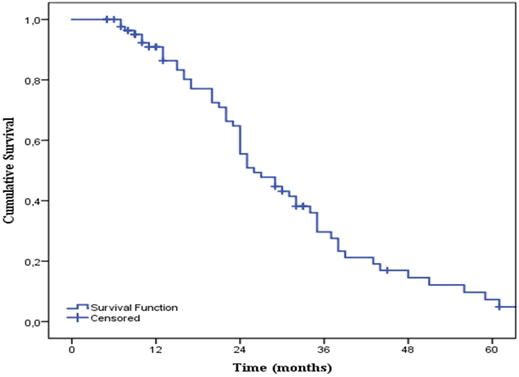
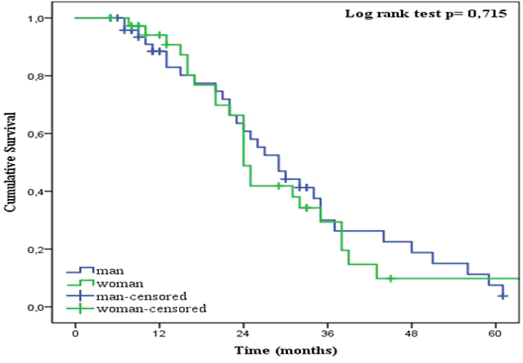
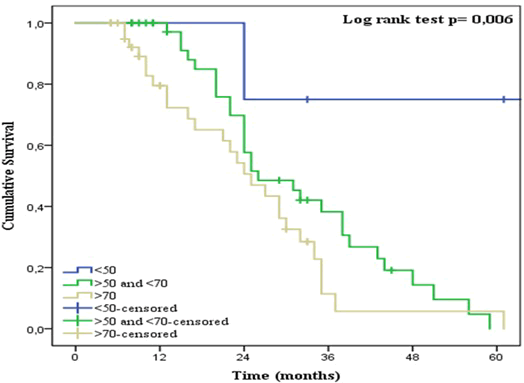
| The patient cohort had a two-year overall survival (OS) of 56% (95% CI 44-68) (Figure 5). The median OS was 26 months (95% CI 20-32). At the time of analysis, 66% (n = 57) of the patients were dead and 34% (n = 30) were still alive. Ninety-one percent (n = 52) of the non-survivals had died from GI-cancer and 9% (n = 5) had died from other causes. For non-survivals older than 70 years and between 50 and 70 years old, 88 % (n = 23) and 93% (n = 27) died from GI-cancer respectively. All non-survival in the youngest patient cohort died from the disease. Males had a higher two-year OS (61%) than females (49%). However, no significant difference was found (p = 0.715) (Figure 6). Significant differences (p = 0.006) were found between the different age groups. As shown in Figure 7, young age was associated with a higher two-year OS of 75%, than for patients between 50-70 years (OS = 58%) or older (OS = 51%). |
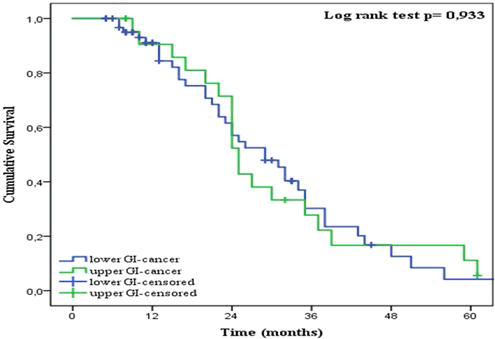
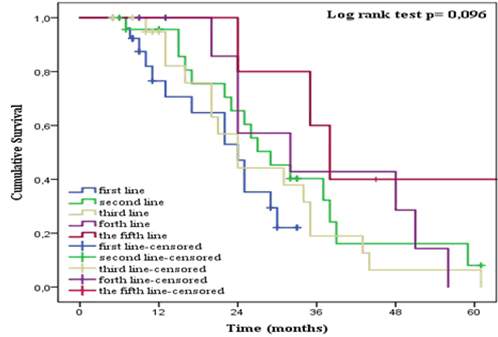
| As shown in Figure 8, no significant difference (p = 0.933) was found between the groups with upper and lower GI-cancer. The two-year OS for upper and lower GI-cancer was 52% and 57% respectively. Patients treated with metronomic Xeloda as the fifth line of treatment fared better than others (OS = 80%) (Figure 9). However, no significant differences were found between the groups (p = 0.096). The two-year OS for patients receiving lowdose Xeloda as the first, second, third and fourth line was 47%, 63%, 44% and 57% respectively. Altogether, significant differences in OS were found for age, but not for gender, treatment line or tumor location. |
|
Safety and quality of life
|
| In total, 76% (n = 66) of the patients reported a good QoL with no bothersome side effects during treatment with metronomic Xeloda. Twenty-six percent (n = 12) of the patients with monotherapy and 22% (n = 9) of the patients with combination therapy experienced adverse effects, which to some extent had an impact on their QoL. The safety profile of metronomic Xeloda is detailed in Table 2. The most common adverse effects were diarrhea (n = 6) and hand-foot syndrome (n = 5). These were generally mild and treated according to conventional routines, but no dose adjustments were needed. Other adverse effects included in this study were skin rashes (n = 3), leg redness and swelling (n = 3), dry mucosal membranes (n = 2), abdominal pain (n = 1) and convulsions (n = 1). No high-grade toxicity was reported and all side effects (n = 21) were of grade I or II. There were no treatment-related deaths. In the youngest patient cohort, 60% (n = 3) experienced treatment-related side effects. The corresponding data for patients between 50 and 70 years and older than 70 years were 22% (n = 9) and 22% (n = 9) respectively. Overall, the treatment was well tolerable with few serious adverse events. Data related to side effects are summarized in Table 3. |
Discussion
|
| GI-cancer is a common cancer form where the primary curative treatment is surgery. Unfortunately, many patients already have a metastatic disease at the time of diagnosis, thus limiting the possibility for tumor resection. In some cases, neoadjuvant chemotherapy can reduce tumor size, thereby making surgery possible. For other patients with unresectable tumors that do not respond to chemotherapy, the intention of treatment often is palliative. The main features of palliative treatment with chemotherapy are to prevent tumor progression and control symptoms without reducing QoL more than necessary. For such purposes, a low-dose continuous treatment approach may offer important advantages over conventional chemotherapy. Phase II studies have demonstrated clinical benefits of metronomic chemotherapy, such as promising tumor control rates and excellent safety profiles [16,17]. In this study, we investigated the efficacy and tolerability of low-dose continuous Xeloda in an extended patient material with metastatic GI-cancer. The patients included were those who received high-grade toxicity to prior treatments or because of their high age or medical contraindications were not considered suitable for conventional chemotherapy. For a large proportion of the patients, all evidence-based treatment alternatives had already been tested. |
| Overall, many patients responded well to the treatment. Two months after initiation of treatment, 38% of the patients had an active but stable disease. Regress of the disease was also seen in 13% of the cases. Regress and stable diseases were seen in patients with both lower and upper GI-cancer. At the time of analysis, 30 patients were alive. Thirteen of those patients remained progression-free under treatment with metronomic Xeloda. These results are comparable with earlier results [6]. Miger et al. did a retrospective study, which included 35 patients with metastatic GI-cancer. Those patients were for the same reasons not considered suitable for evidence-based medicine and received metronomic Xeloda (500mg × 2). Two months after initiation of treatment, about 46% (upper GI-cancer) and 39% (lower GI-cancer) of those patients were considered to have a stable disease. Regress was seen in 31% of the patients with upper GI-cancer. |
| The whole patient cohort had a high median survival of 26 months and a two-year survival rate of 56%. Survival was related to age, but no significant differences were found for tumor location, gender or treatment lines. The high OS for the youngest patient cohort is impressive, but it is a small number of patients, selected by chemosensitive tumors where all other treatment schedules have failed. Still, the patients had a high performance status and were highly motivated for treatment. To our knowledge, there are no suitable randomized studies available to compare our survival data with. However, the CALBG study which included more than 1000 patients with metastatic CRC had a median survival of 30 months [33]. In that study, patients were treated with fluorouracilbased chemotherapy in combination with either bevacizumab or cetuximab as the first line of treatment. |
| One of the main concerns regarding conventional chemotherapy is that the drugs are used at doses near or at the MTD, which often results in toxic drug-related adverse events [5]. In this study, we also intended to investigate QoL and side effects during treatment with metronomic Xeloda. As much as 76% of the patients reported a good QoL during the treatment with Xeloda. For those patients, the treatment had a minimal or none impact on daily life. None of the reported adverse effects were of toxicity-grade III or IV. Most frequent side effects were handfoot syndrome and diarrhea. According to other reports, such side effects are very common for capecitabine [34]. Our results show that low-dose capecitabine is well tolerated, hence the long treatment durations and few reported side effects. These results are consistent with most clinical trials, which have shown that metronomic chemotherapy is very tolerable and high-grade toxicity is rare [14]. |
| Metronomic Xeloda has most of all been examined in breast cancer, but also for advanced CRC [14,35]. In other studies at our clinic, metronomic Xeloda has been investigated with remarkable results in patients with metastatic esophageal cancer and CRC [6,9,14]. In one of the reports, the patients were diagnosed with mucinous adenocarcinoma in the transverse colon with three liver metastases [14]. Since surgery was not considered possible and neoadjuvant chemotherapy with fluorouracil, folinic acid and oxaliplatin failed due to several infections, the patient was referred to palliative care. Treatment consisted of metronomic Xeloda (500 mg x 2) daily together with Avastin every other week. After four months, the tumors had regressed and the patient thereafter underwent radical surgery. Nine months after the operation, there were still no signs of recurrence. An interesting feature of this case is that mucinous adenocarcinomas have been shown to have a poorer response to both chemotherapy and chemoradiotherapy compared to non-mucinous adenocarcinomas [36,37]. |
| Metronomic chemotherapy is believed to act by other mechanisms than classical chemotherapy. Within the classical chemotherapeutic regimens, the cytostatic drugs often are administered intravenously, at high doses near the MTD. The intention is to treat cancer by killing or inhibiting the rapidly dividing cancer cells. Traditional chemotherapeutic agents act as enzyme inhibitors, false metabolites, mitotic inhibitors or alkylators in order to cause DNA-damage or inhibit mitosis [19]. To our knowledge, the mechanisms of metronomic cancer treatment are not fully understood. At first, metronomic chemotherapy was mainly thought to achieve cancer control by primary targeting tumor angiogenesis, but recent studies have revealed that metronomic chemotherapy might be a multitargeted therapy [5]. The anti-angiogenic properties of low-dose chemotherapy have been demonstrated in vivo and in vitro [11-13]. These mechanisms include selective inhibition of migration and proliferation of endothelial cells, decreased levels of bone marrow-derived endothelial progenitor cells and increased levels of thrombospondin-1, an endogenous inhibitor of angiogenesis [8]. Studies have shown that metronomic chemotherapy may also stimulate the immune system and induce tumor dormancy [10,14]. The immune stimulatory effect is thought to be mediated by inhibition of Tregs and activation of dendritic cells. Metronomic chemotherapy can induce tumor dormancy by three different methods, which include immune-surveillance, suppression of angiogenesis and apoptosis of malignant cancer cells [5]. Since low-dose continuous chemotherapy offers a high preference for oral formulations and do not require hospital admission, it has shown to have a high degree of acceptance by both patients and physicians [35,38]. Oral formulations are cheaper, easier to administer and do not require hospital admission. From a cost perspective, metronomic treatment may therefore be more costeffective alternative compared to conventional chemotherapy [15]. |
| Finally, our results show that metronomic Xeloda seems be a promising alternative for motivated patients in a good general condition when evidence-based regimens are not considered suitable or have failed. However, patient data are collected retrospectively, which requires great caution when interpreting the results. More research is needed in order to better understand the potential promise that metronomic Xeloda might offer. Hopefully, this study may provide with inspiration and the basis for a future randomized prospective study within this research field. |
Conclusion
|
| Palliative treatment of metastatic GI-cancer is often limited to a few therapeutic options. Where the aims of treatment are to achieve tumor control and a good QoL, a continuous low dose approach with Xeloda, probably acting through different mechanisms, may offer a therapeutic alternative. Our results show that metronomic Xeloda is efficient and safe, measured in median survival, side effects, QoL and response. Both tumor regression and stable diseases were observed. Future randomized prospective studies are needed in order to fully understand the clinical benefits of metronomic treatment. |
Author contribution
|
| DG participated in the design of the study performed the statistical analysis and drafted the manuscript. MA designed the study and helped to draft the manuscript. All authors read and approved the final manuscript. |
Tables at a glance
|
|
|
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4A |
Figure 4B |
 |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
|
| |
| |















