Introduction
A 56-year-old patient who experienced a stroke-like episode of sensory deficit on the left hemi-body, spent 2 years pullulating different consulting rooms in the pursuit of recovery. He was referred to a psychiatrist. He was seen in private medicine. He was diagnosed CVA of unknown etiology and anxiety-depression disorder. 18 months after having initiated the neuroimaging study, a “contrast uptake” was found in the hypodensity, which was interpreted as being ischemic. The diagnosis was not modified. The patient died 6 months later of an untreatable huge volume expansive process, diagnosed with B-cell lymphoma of the brain.
We have analyzed the explorations and evolution during those two years.
Current health condition
A 56-year-old male patient, who consults doctors, since after having accidentally fallen over a steel table, he presented a numb episode on the left side of the face and the left hand. He also observed muscle weakness in such hand. After a month, he went to the emergency room, and a CAT scan showed signs of leucoaraiosis (image 1), although when observed a posteriori, it is possible to see small hypodense injuries in the dorsal thalamus (arrow).
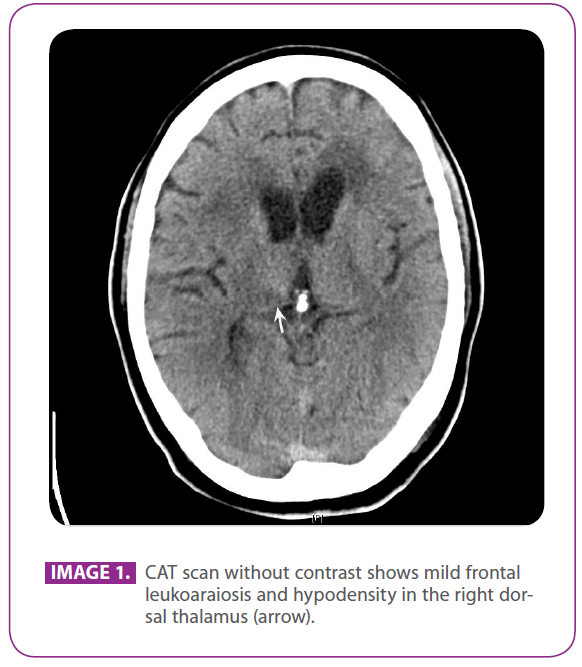
Image 1: CAT scan without contrast shows mild frontal leukoaraiosis and hypodensity in the right dorsal thalamus (arrow).
Subsequently, during the following weeks, the sensory symptoms spread to the left arm. Previously, as a child he had suffered from poliomyelitis with residual palsy (2/5) in the left lower extremity. He smoked 20 cigarettes per day; and he was a moderate drinker. A few years before, he had suffered from depression. As a consequence of a traffic accident he had right tibia and fibula comminuted fractures with secondary osteomyelitis and an relevant venous affection. No toxic contacts were referred.
His father was from Germany and died of leukemia. He was referred to the regional hospital and he was diagnosed of ischemic CVA. He was prescribed to take Aspirin 300mg and Omeprazole 20mg. During the following months the sensory symptoms got worse. He was also clumsy when walking and it was difficult for him to move the left arm. He had to use crutches. He had difficulty to coordinate the movements of the left arm and leg.
He never complained about cephalalgia, dizziness or vertigo. Later on, he had difficulty to pronounce words and he was unable to button and tie the shoelaces. He was given brain MRI scans (image 2) compatible with ischemic injuries in the right dorsal thalamus region (arrow).
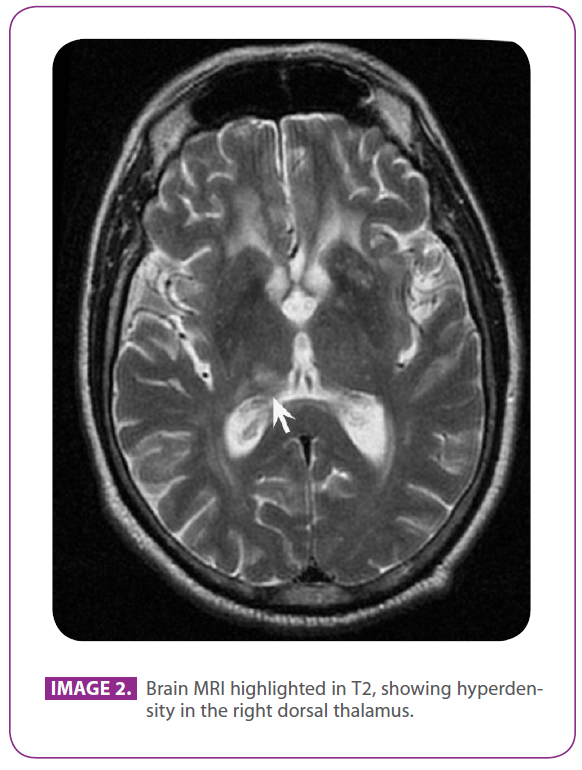
Image 2: Brain MRI highlighted in T2, showing hyperdensity in the right dorsal thalamus.
9 months after the symptoms started the sensory symptoms got worse, he consulted a neurologist who found the following in his exploration: blood pressure of 120/80. He had pseudoatetosis and dystonic posture of the left hand. A scar in the right anterior tibial region, which was hyperpigmented and not epitalized. Atrophic and arreflexic left leg caused by the poliomyelitis. He was conscious, oriented and collaborative. Normal speech. Normal Confrontation campimetry. Fundoscopy: pale papillae. Cranial nerves: normal oculomotors, facial, hypoglossal and vagus nerves. Normal Photomotor reflex. Increased tone on the left side with mild brachial palsy (3-4/5) and higher crural (2-3/5). Higher myotatic reflexes on the right side (sic). Lower algesic sensibility on the left side and very low vibration sensibility on the left side. Coordination: dismetry in left extremities. Unstable barany. Clumsy walking, need of crutches. Cardiac auscultation and rhythmic laterocervical had no bruits.
Patient was prescribed to take Oxcarbazepine 600 mg every 12 hours; he experienced a mild recovery for a while. 12 months after the symptoms started, he consulted another neurologist, who decided to hospitalize him in view of the prevailing worsening sensory symptoms. During the hospitalization, the following tests were conducted:
• Blood count, hemogram, VSG, coagulation and biochemical factors: normal.
• Urine test: normal.
• Proteinogram and Immunoglobulins in blood: normal.
• Lumbar puncture: 1 erythrocite and 1 leucocyte/mm3. Glucose 69 mg, total proteins: 39,8 mg/dl. Cytology: monocytoid cells and lymphocyte with non relevant histological alterations. Cytomegalovirus PCR: negative. Negative cultures including mycobacterias. Ogliclonal bands: positive in LCR and in serum, “in mirror” pattern. Serology for brucella, lues and borrelia: negative.
• Carotid duplex: right axis: hyperechogenic structure with hypoechoic nucleus of 16,2 x 3,2 mm at the level of the right common carotid artery, but it does not provoke relevant hemodynamic alterations. Left axis: normal. Permeable vertebral arteries with normal flow and direction, but globally low speeds. Using a transcranial probe, the basilar is probed, with a depth of 75 to 90 mm, observing a resistive curve with diastolic collapse.
• Multimodal evoked potentials: alterations in the somestesic stimuli of the left extremities.
• Electroneurogram and electromyogram: diffuse chronic neurogenic symptoms, in left L4-S1 myotomes, with prevailing left L5 motor unit loss. No symptoms of active axonal damage. Entrapment of the two median nerves in the carpal tunnel, very high on the left side and high on the right side.
• Electroencephalogram: normal fundamental value activity, with no focal or generalized overadded signs.
• Head MRI with and without contrast: multiple hyperintense lacunar images in the subcortical white matter of the frontal lobes, semioval and periventricular centers in relation to senile changes of the white matter of ischemic-degenerative origin. There is a hyperintense area in the right semioval center and the right radiate crown, which shows a mild restriction of the compatible diffusion with the ischemic injury in the recovery phase, found in the right middle cerebral artery region. Subarachnoid space enlargement and ventricular system enlargement in relation to the discrete cortico-subcortical atrophy. Mild enhancement (images 3 and 4, arrows).
• Intra and extracranial angioresonance: normal.
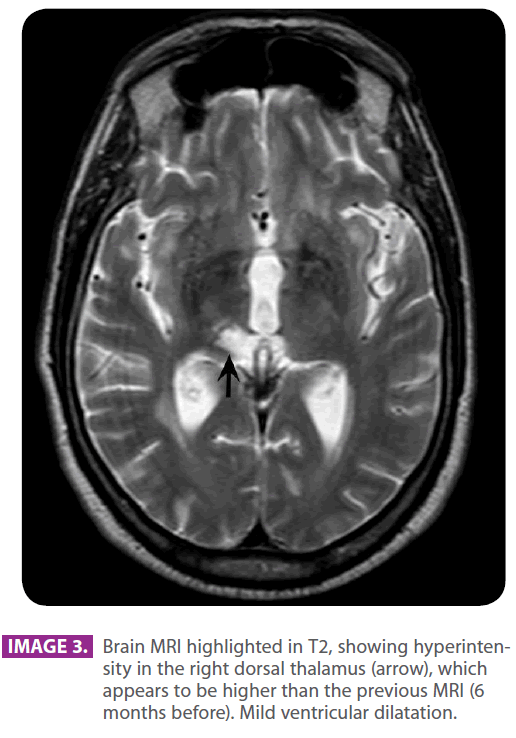
Image 3: Brain MRI highlighted in T2, showing hyperintensity in the right dorsal thalamus (arrow), which appears to be higher than the previous MRI (6 months before). Mild ventricular dilatation.
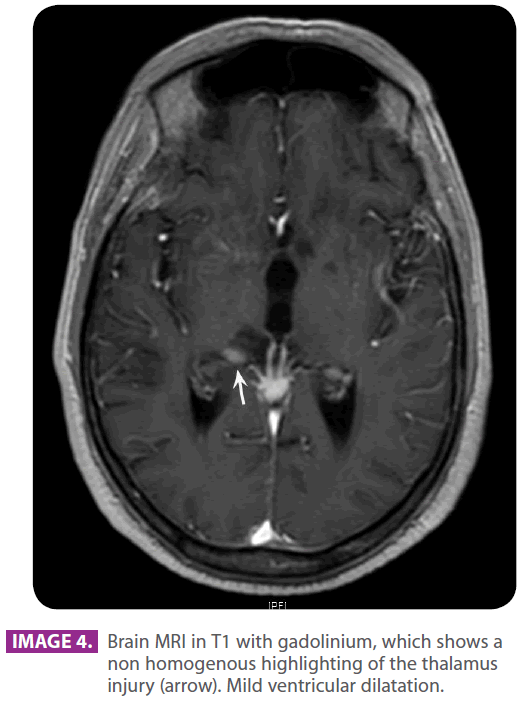
Image 4: Brain MRI in T1 with gadolinium, which shows a non homogenous highlighting of the thalamus injury (arrow). Mild ventricular dilatation.
The antiaggregation continued, introducing Pregabalin 75 mg every 12 hours.
Two months later and 14 months after the symptoms started, the patient complained that sensibility had got worse and there was a kind of left extremity movement loss. Patient was referred to a psychiatrist, the dose of Pregabalin was increased to 150 mg every 12 hours, and benzodiazepines and escitalopram were included.
Eight months later and 22 months after the symptoms started, the family consulted their primary care physician for sleepiness and slowness in his reactions with a mild confusional state. The dose of Pregabalin was reduced to 75 mg ever 12 hours.Since he did not experience any recovery, he was referred to the emergency room, where he was given a CAT scan, which showed an intraaxial expansive lesion with relevant mass effect (image 5). Patient was referred to neurosurgery. Patient experienced bradypsychia and sleepiness. Dysarthric speech. Patient had a mild headache, which increased with coughing. He could not keep himself in sitting posture. He was given a brain MRI (image 6) and then he underwent a stereotaxic biopsy, which found histological and immunohistochemical results compatible with B-type lymphoma.
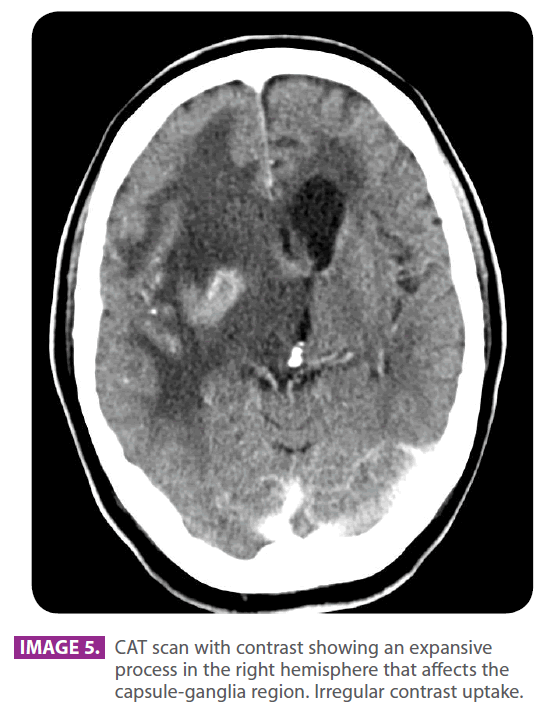
Image 5: CAT scan with contrast showing an expansive process in the right hemisphere that affects the capsule-ganglia region. Irregular contrast uptake.
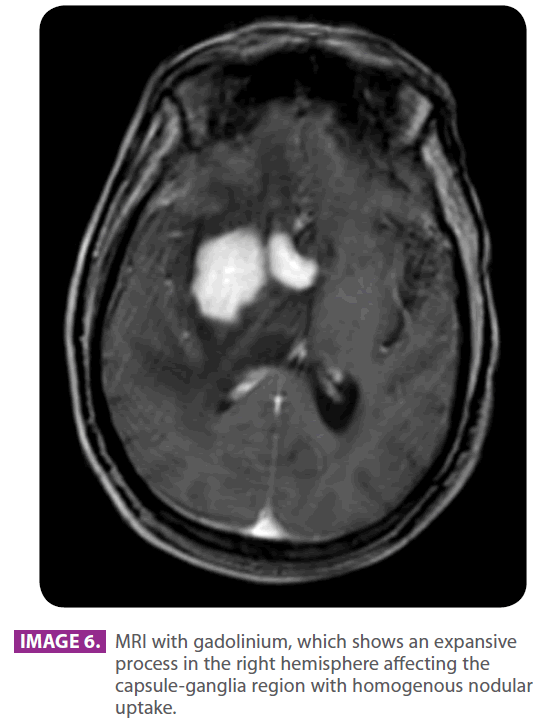
Image 6: MRI with gadolinium, which shows an expansive process in the right hemisphere affecting the capsule-ganglia region with homogenous nodular uptake.
An extension study did not find any extra brain disorders. Patient died a few days later of aspiration pneumonia. 24 months have passed after the symptoms had started. Autopsy was not performed.
Comments
Thalamic syndrome
The classic Dejerin Roussy thalamic syndrome is characterized by:
• Extremity and contralateral face paresthesia, sometimes with a few sensory disorders.
• Colateral hemi-palsy, generally temporary.
• Cerebellar syndrome in such extremities.
• Distonic postures with choreic or athetotic movements (thalamic unstable hand).
• Pains a few weeks or months later. Some patients may also present Claude Bernard-Horner syndrome (hypothalamic disorder).
The most frequent cause is vascular ischemic disorder. The infarction mainly occupies the sensory nucleus and other thalamic and partially the internal capsule. The deep branches of the posterior cerebral artery are interpedunculars, thalamoperforating or thalamoparamedian and the thalamogeniculate artery. The softening caused by the arterial obstruction of the two latter generates the classic thalamic syndrome.
The Dejerin Roussy thalamic syndrome was described for those authors in 1906. They described a hemi-palsy syndrome, which recovered fast along with the persistent choreic, ataxic, hyperesthesia and paroxistic acute pain on the hypoesthesic side. Sometimes, hand dystonia is associated. A mild hypoesthesia is usually associated. Dysesthesia and unpleasant cold, tiresomeness and heat feelings are very common.
A tiresomeness feeling may be experienced in the extremity. The syndrome might even appear a few months after the ischemic accident. Hyperpathia might be spontaneous or caused by stimuli, constant or paroxistic, temporary or permanent. Pain might vary from pins and needles to an unbearable discomfort. They correspond to the posterior lateral and ventral posterior middle nucleus of the thalamus. It has also been associated to lacunar infarctions.
Primary lymphoma of the brain
The primary lymphomas suppose 1-7% of all intracranial neoplasms. They are usually B-lymphocyte lymphomas. Its incidence has increased in the last decades in immunocompetent patients (experienced in 50-60 year-old patients) and immunodepressed patients, especially for HIV and immunosuppressed patients1.
The neuroimage and evolution aspects differ between those found in immunocompetent patients and immunodepressed patients [1].
They tend to be focal masses, relatively well circumscribed or they might act as infiltrants. Central multiple injuries are very common: basal ganglia, periventricular region and corpus callosum.
In the CT scan they are isodense or moderately hyperdense, with intense enhancement and homogenous with contrast [2]. They are usually found in the periventricular white matter and basal ganglia and they are homogenously and intensely enhanced with contrast. They might go through the corpus callosum and spread through the ependimary surface [2]. In the magnetic resonance they are iso-hypointense in T1 and T2 sequences and hyperintense in FLAIR sequences. They present restriction in the diffusion (high signal) [2,3].
In the scarce lacunar infarction cases with pure sensory frames, arteriosclerosis and lipohyalinosis of small arteries have been observed [4].
Tendency is to recovery with time.
From the anatomopathological point of view, it is characterized by small lymphocytes concentrated in a perivascular pattern1.
2204
References
- Bataille B et al. Primary intracerebral malignant lymphona: report of 248 cases. J Neurosurgery. 92: 261-6. 2000.
- Linfoma primario del SNC. En: Los 100 diagnósticos principales en cerebro. Serie Radiológica clínica. Editores: Osborn, Blaser y Salzman. Elsevier. Madrid. 2004. pg 174-177.
- Orrison WW, Blaine L. Hart. Tumores cerebrales intraxiales. En: Neurorradiologia. Editor: W.W. Orrison Jr. 1ª edicion. Harcourt. Madrid.2001. pg.606.
- Mohr JP, Marti-Vilalta JL. Lacunes. En: Stroke. Editores: Barnett HJM, Mohr JP, Stein BM, Yatsu FM. Third edition. Churchill Livingstone. New York. 1998. pg 608-610.











