Nafees Uddin Chowdhury1, Tasdik Farooq2, Shahanshah Abdullah2, Ahmed Shohrawar Mahadi2, Md Mehedee Hasan3, Tasfiq Zaman Paran2, Nahid Hasan2, Md Mohabbulla Mohib2, Md Abu Taher Sagor2* and Md Ashraful Alam2
1Department of Prosthodontics, Kumudini Women's Medical College, Dental Unit, Mirzapur, Tangail, Bangladesh
2Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh
3Department of Pharmacy, State University of Bangladesh, Dhaka, Bangladesh
*Corresponding Author:
Md Abu Taher Sagor
Department of Pharmaceutical Sciences
North South University, Dhaka-1229, Bangladesh
Tel: +8801719130130
Email: sagor2008nsu@gmail.com
Received date: July 11, 2016; Accepted date: September 28, 2016; Published date: October 03, 2016
Citation:Chowdhury NU, Farooq T, Abdullah S, Mahadi AS, Hasan MM, et al. (2016) Matrix Metalloproteinases (MMP), a Major Responsible Downstream Signaling Molecule for Cellular Damage - A Review. Mol Enz Drug Tar 2:3.
Copyright: © 2016 Chowdhury NU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Cellular damage; Downstream molecules; Inflammation; Apoptosis; MMP
Abbreviations
TRADD: TNFRSF1A-associated via death domain; FADD: Fasassociated protein with death domain; Rac1b: Alternative splicing of Rac1 generates Rac1b; BAX: BCL2-associated X protein; Bcl-2: B-cell lymphoma 2; CHEK2: Human gene checkpoint kinase 2; MDM2: Mouse double minute 2 homolog also known as E3 ubiquitin-protein ligase Mdm2; PERK: Protein kinase RNA-like endoplasmic reticulum kinase; ATF4: Activating transcription factor 4; APAF1: Apoptotic protease activating factor 1; IAP: The inhibitor of apoptosis
Introduction
Diabetes, obesity and hyperinsulinemia may lead to liver damage [1], heart dysfunctions [2] and end stage renal diseases [3]. Evidences also revealed that the rate of cancer development is also increased among patients throughout the world [4]. All together, the rate of mortality and morbidity due to metabolic diseases and cancer have been increased in an alarming rate [5]. In most of the cases, it was observed that downstream small molecules such as interleukin-1β, tumor necrosis factor-α, activator protein-1, hypoxia induced factor, macrophage inflammatory protein and nuclear factor-κβ were found responsible for cellular damage and progression of multiple pathologic conditions [6]. Conversely, recent studies also showed that matrix metalloproteinase plays a significant role as a signaling molecules [7]. MMPs family has been identified for the development of several diseases [8] such as skin diseases [9], diabetic complications [10], atherosclerosis [11], end stage renal diseases [12], fibrosis [13], vascular dysfunctions [14], inflammation [15], chronic hepatic diseases [16], iron overload [17], pulmonary emphysema [18], cerebral ischemia [19], myocardial infarction [20], angiogenesis [21], cancer [22], apoptosis [23] and several other diseases.
Polymorphism in MMPs may promote alteration of several gene regulation that consequently change the genetic profile [24]. Similarly, it has been reported that smoking cigarettes may induce MMPs and in turn accelerates the development of several types of cancers [25]. One recent report evaluated that MMP-13 role in the development of atherosclerosis via AKTERK mediated pathway and explained the migration of vascular smooth muscle cells (VSMCs) [26]. Another study also investigated that neuronal matrix metalloproteinase-9 which showed that it may be responsible for several neurodegenerative processes by activating ER stress in ALS motor neurons [27]. Expression of MMP-2/MMP-9 were also observed in experimental acute kidney allograft rejection [28]. The members of MMP family are also identified as proinflammatory signaling molecules in various studies [29]. In addition, MMPs are further responsible for regulation of several growth factors like VEGF and EGF which ultimately damage vascular system [30]. Furthermore, study also revealed that MMP-8 delays wound healing process in mice [31].
In the last three decades, experiments on this protein family have been largely investigated the multiple up-regulated and down-regulated pathways for cellular catabolism, production and renewal of various biochemical processes. As MMP family members are playing central role in multiple diseases progression, development and establishment of MMP inhibitors may have been a primary concern for the current ongoing research in this field. It has been noticed that doxycycline non-selectively suppresses MMP function in patients suffering abdominal aneurysm [32]. Over 60 other MMP inhibitors are currently being investigated to develop an active component against MMP mediated dysfunctions [33]. MMP inhibitors may play a beneficial role by abating several cellular signaling pathways like cellular inflammation, vascularization, fibrosis and apoptosis. Therefore, this review will evaluate the possible molecular mechanisms induced by MMP family members and few possible treatment approaches in various pathophysiological conditions.
Matrix Metalloproteinases and their Family Members
Generally, enzymes are known as catalysts which speed up a reaction. Thus, MMPs catalyze several pathways for cell migration, invasion, regulation and proliferation [34]. It was previously thought that MMPs are present in tissues to help degrading several regulatory components of the extracellular matrix and other basement membrane. However, recent studies explored that they may also play significant role as a prime regulator for various signaling networks. These proteins also have been known as the member of metzincin group of proteases which contribute to the conserved zinc-binding motif inside their catalytic binding sites [35]. MMPs are mainly triggered by several factors and chemicals such as oxidized glutathione, chaotropic agents, thiol-modifying agents, sodium dodecyl sulfate and free radicals which interfere with cysteine- Zn2+ of the cysteine-switch motif (Figure 1) [36].
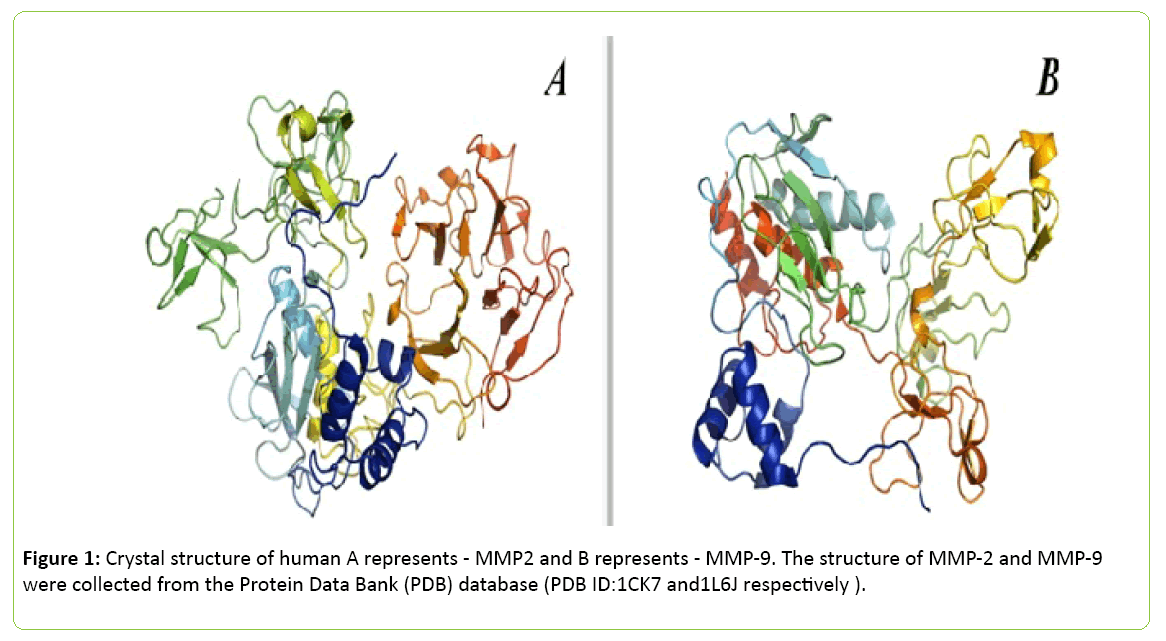
Figure 1: Crystal structure of human A represents - MMP2 and B represents - MMP-9. The structure of MMP-2 and MMP-9 were collected from the Protein Data Bank (PDB) database (PDB ID:1CK7 and1L6J respectively ).
The images were processed using PyMol (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC) and UCSF Chimera package. Inspired from [37,38]. Several members of MMP family also have been isolated. In 1962, the concept of MMP was first established [39], since then more than 30 active members of this family have been characterized successfully. Several sub-classes have been drawn for identification like MMP-1, MMP-8 and MMP-13 belong to collagenases subclass; MMP-2 and MMP-9 belong to gelatinases subclass; MMP-3, MMP-10 and MMP-11 belong to stromelysins subclass; MMP14, MMP-15 and MMP-17 belong to membrane-type MMPs; MMP-7 and MMP-26 belong to matrilysins subclass (Table 1) [40].
| Group |
Protein name |
Sub cellular location |
Substrate(s) |
| Collagenases |
MMP1 |
Secreted |
Collagen types I, II, III, VII, and X |
| MMP8 |
Cytoplasmic granules and secreted |
Collagen types I, II, III, VII, and X |
| MMP13 |
Secreted |
Collagen types I, II, III, VII, and X |
| Gelatinases |
MMP2 |
Secreted, membrane, mitochondria, nucleus |
Gelatin type I, Collagen types I, II, III, VII, and X |
| MMP9 |
Secreted |
Gelatin types I and V, Collagen types IV and V, and Fibronectin |
| Stromelysins |
MMP3 |
Secreted |
Gelatin types I, III, IV and V, Collagen types III, IV, IX and X, Laminin, Febronectin, pro-MMP1 |
| MMP10 |
Secreted |
Gelatin types I, III, IV and V, Collagen types III, IV, IX and X |
| MMP11 |
Secreted |
α-1-antiprotease |
| Matrilysin |
MMP7 |
Secreted |
Gelatin types I, III, IV and V, and Febronectin |
| MMP26 |
Secreted |
Gelatin type I, Collagen type IV and Febronectin |
| Enamelysin |
MMP20 |
Secreted |
Aggrecan |
| Metalloelastase |
MMP12 |
Secreted |
Elastin |
| Membrane-type (MT) MMPs |
MMP14 |
Membrane |
Pro-MMP2 |
| MMP15 |
Membrane |
Pro-MMP2 |
| MMP16 |
Membrane |
Collagen type III and Febronectin |
| MMP17 |
Membrane |
Fibrin |
| MMP24 |
Membrane |
N-cadherin(CDH2) |
| MMP25 |
Membrane |
Pro-MMP2 |
| Others |
MMP19 |
Secreted |
Collagen type IV, Laminin, nidogen, Nascin-C isoform, Fibronectin, and type I gelatin. |
| MMP21 |
Secreted |
α-1-antitrypsin |
| MMP23 |
Cell membrane, ER membrane |
- |
| MMP27 |
ER membrane |
Fibronectin, Laminin, Gelatins and Collagens |
| MMP28 |
Secreted |
- |
Table 1: An overview of MMP family members.
Matrix Metalloproteinases and Inflammation
Inflammation is the signaling process for a cellular regulation which denotes imbalance inside organ or tissue. Pain, swelling, redness or rash and fever are generally observed in acute cases of inflammation but often noticed cellular death if the process persists for a longer period of time [3,41,42]. Furthermore, MMP family members are also known as inflammatory cytokines [43]. The outcomes of inflammation were cell necrosis, damage and cellular death in various animal model studies [44,45]. It has been noticed that MMP-9 is highly responsible molecule in neuro-degenerative diseases which further inducing the activation of pro-inflammatory factors such as PKCs, ROS, ERK1/2, PI3K/Akt, NF-κB, and AP-1 (Figure 2) [46]. Several experiments also noticed that MMP-1 stimulates VEGFR2 expression which further promotes endothelial proliferation via activation of NF-κβ and protease activated receptor-1 (PAR-1) [47,48]. In rodents, MMP-2 and MMP-9 significantly increased inflammatory cytokines such as TNF-α, LI-6, IL-8, IL-1β, MIP-1α and GROα in the lung when the animals were treated with LPS. This study also revealed that LPS administration increased tissue MPO, neutrophil, and eosinophils level in lung [49]. Studies also suggested that family members of MMP regulate chemokines activities. The mechanism of controlling chemokines has been explained as the cleavage progressed by MMPs [50,51]. Another study has demonstrated that MMP-2 directly cleavages motif of monocyte-chemotactic protein (MCP) in a yeast two-hybrid system which is further known as CCl7 or MCP3 [52]. Other studies also explored that MMP-7 plays as a potent pro-inflammatory cytokines in lung. Accumulation of neutrophil reflux and oxidative burst cause activation and production of mucosal immunity along with epithelial migration that eventually possess mortal damage [52,53].
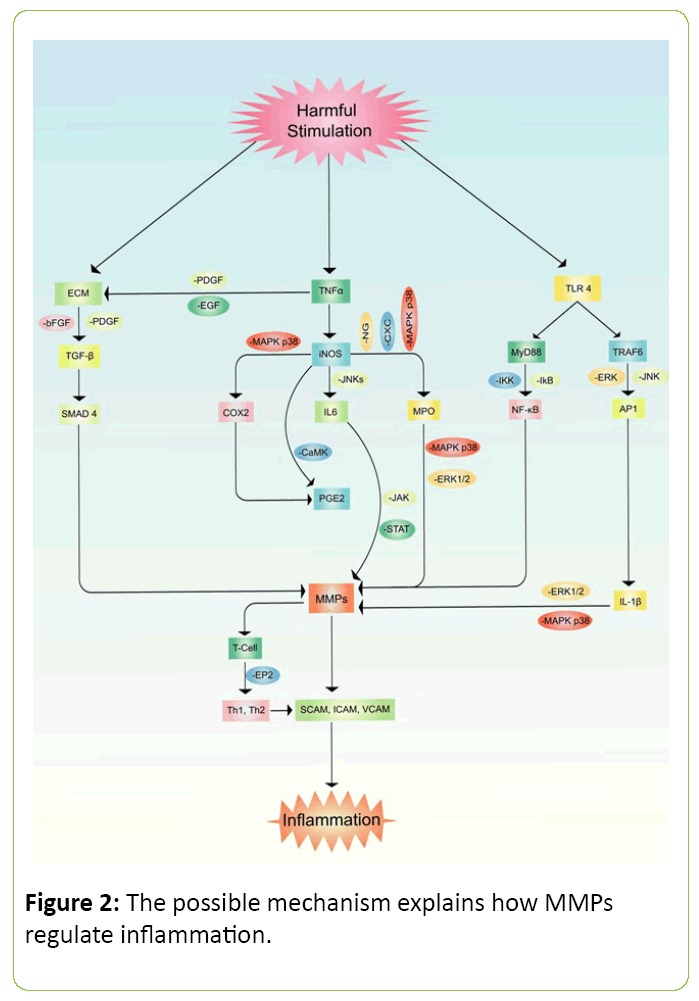
Figure 2: The possible mechanism explains how MMPs regulate inflammation.
Matrix Metalloproteinases to Induce Cytokines Production
MMPs not only stimulate immune cells but also induce several other cytokines production and activation. Evidences have suggested that MMP-2 activates c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) which further activates transcription factor for NF-κβ in rat HSCs [54]. The expression of MT1-MMP and proMMP-2 were also noticed in tumor cells [55]. Iron is generally stored inside a cell and highly regulated by hemeoxygenase (HO) [41]. In several disease conditions, hemeoxygenase is degraded and resulting in iron overload which further assists in the development of multiple pathogenesis [56]. MMP-1, MMP-2 and MMP-8 have strongly been blamed to degrade the HO regulation which initiate iron deposition [57,58]. Previous investigation also suggested that expression of MMP-2 and MMP-9 may increase the production of HLA-DR antigens concentration on plaques by interacting with COX-2/mPGES [59]. Another experiment found that MMP-9 helps in the expression of hematopoietic CD34+/CXCR4+ stem cells that is linked to wound healing capability [7].
Matrix Metalloproteinases and Apoptosis
Apoptosis is a biological phenomenon where cells generally are programmed to die without inducing inflammation. Sometimes body initiates this process to replace old cells. This process can be harmful when a hazardous molecule invades inside a biological system and starts apoptosis where it is not necessary [60]. MMPs family members have been intensively blamed to initiate this process which results in cellular death [61]. It is mostly suggested and evaluated that the process of apoptosis is taken through the production of caspase proteins [62]. A possible mechanism has been proposed for apoptosis via MMP in Figure 3. Generation of free radicals, along with up regulation of MMP-9 from BK-challenged brain astrocytes trigger brain cell apoptosis. This study also suggests that MMP-9 mediated pathway may severely damage neural cell [63]. N-cadherin, a cell survival protein was found less due to MMP-7 expression on human atherosclerotic plaques which ultimately reduces the cell survival rate [64]. Other study also observed that MMP mediated cerebral endothelial cell death may occur due to over expression of caspase activity [65].
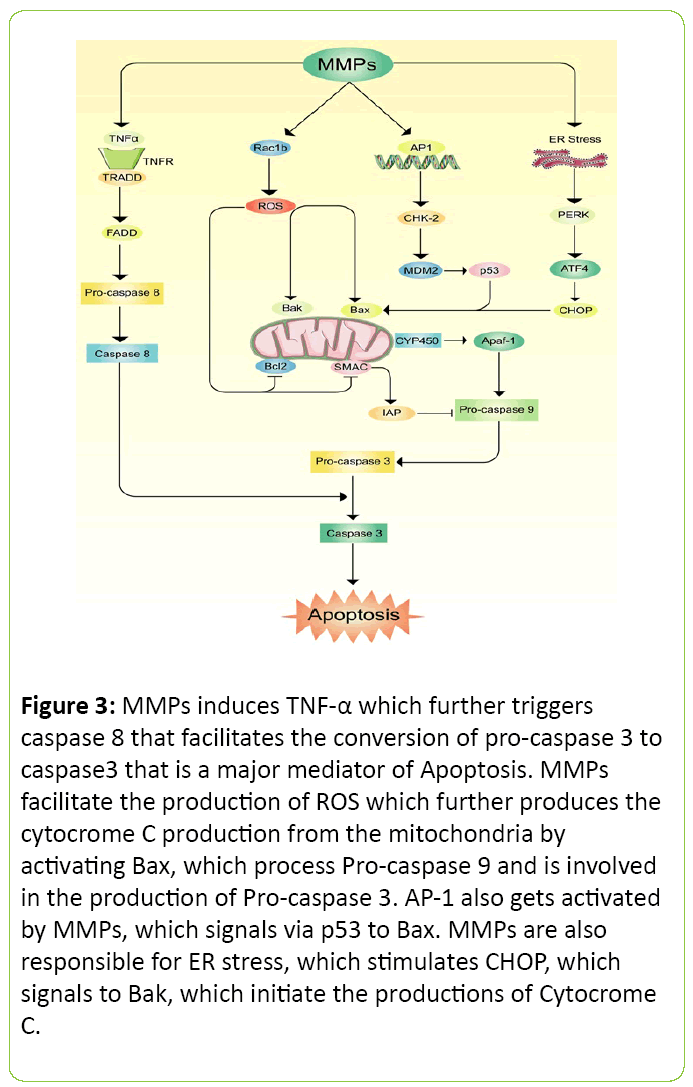
Figure 3: MMPs induces TNF-a which further triggers caspase 8 that facilitates the conversion of pro-caspase 3 to caspase3 that is a major mediator of Apoptosis. MMPs facilitate the production of ROS which further produces the cytocrome C production from the mitochondria by activating Bax, which process Pro-caspase 9 and is involved in the production of Pro-caspase 3. AP-1 also gets activated by MMPs, which signals via p53 to Bax. MMPs are also responsible for ER stress, which stimulates CHOP, which signals to Bak, which initiate the productions of Cytocrome C.
Matrix Metalloproteinases and Cancer
Cancer is a group of various metabolic disorders which explains abnormal or uncontrolled cell proliferation that may spread to several other organs [66]. In the recent year smoking cigarette, consumption of alcoholic beverages and chewing tobacco have been identified as the main reasons for the development of cancer [67]. Less or no physical activity, taking high fructose containing beverages, environmental exposure, viruses like hepatitis C or HPV and genetic predisposition also play major role in cancer which may lead to death globally [68]. According to American Cancer Society, 589,430 people died of cancer and 1,658,370new people were diagnosed with cancer in USA in the year 2015 [66]. It is also projected that this figure would be around 22 million in 2030 and the treatment cost will be out of reach to the poor and middle class people [69]. The total cost of cancer treatment and management were estimated more than 1.6 trillion US dollars globally in 2010 [70].
The relationship between cancer and MMP is very strong. Several studies have been investigated that the expression of MMP family members found high in the cancer subjects [71,72]. The expression of MMP-2 and MMP-9 have been targeted in the primary stage of colorectal cancer in mice [73]. Similarly, Membrane type 1-matrix metalloproteinase (MT1- MMP, MMP-14) is also co-related with cancer invasion and metastasis. This investigation suggests that MMP may trigger TGF-β that further induce CUTL1 and subsequently, of Wnt5a through paracrine mediated mechanisms [74]. MMP-9 also has been identified in tumor invasion and metastatic diffusion, including bone marrow and can be found in brain cancer and targeted as a potential biomarker for breast cancer [75]. Furthermore, MMP mediated cancer is generally initiated through inflammatory cytokines [76].
Possible Treatment Approaches
Evidences suggested that the treatments of several diseases are getting difficult due to interfering role by MMP family members. Inhibiting MMP can be a good approach to reduce several pathogenesis like chronic inflammation and cancer [77]. Both natural and synthetic components are being aggressively focused against MMP family members [78]. Zn2+- chelating hydroxamate has been observed effective in the development of MMPI as this carries superior ΔG values. This helps to bind around Zn2+ [79,80]. Signaling molecules such as MAPK or p38MAPK have been found responsible in many diseases. So, blockage of these kinases can be a good way to fight against such pathogenesis [6]. Natural product like naringen down regulated MMP-2 and MMP-9 by reducing signaling of MAPK in Human glioblastoma cell lines (Table 2) [81]. Another study exposed inhibitory activity of MMP-3 by reducing PI3K-Akt signaling pathway when the cell was treated with resveratrol [82]. Gallic acid, a very potent antioxidant observed effective in oral cancer by mainly reducing FAK, PKC, RhoA and NF-κβ [83]. Kaempferol, another phenolic acid, reduced c-Jun activity and phosphorylation of ERK1/2 expression by controlling MMP-2 in human tongue squamous carcinoma cell line [84].
tbody>
| Name of Molecule |
Model |
Mechanism |
Reference |
| Cordycepin |
Rat aortic smooth muscle cells |
Inhibits MMP-13 by blocking migration of vascular smooth muscle cells by preventing Akt and ERK-dependent regulation |
[87] |
| Naringin |
Human glioblastoma cell lines U87 |
Down-regulatesof the expression of MMP-2, MMP-9 by attenuating the MAPK signaling pathways including ERK, JNK and p38 |
[81] |
| DX-2400 |
HT-1080 cells |
Blocks MMPs expression by inhibiting proMMP-2 processing. |
[85] |
| Ac-LEHD-cmk, |
Neonatal cardiomyocytes |
Blocks the MMP-2 activity by reducing TnI proteolysis and hypoxia-reoxygenation |
[88] |
| Fisetin |
Male C57BL/6 mice |
Blocks pro-MMP-2 and active MMP-2 activity |
[89] |
| Solamargine |
HepG2 cells |
ReducesProMMP-2 and ProMMP-9 levels in the cytosol thus blocks the expression of MMPs |
[90] |
| Caffeic acid phenethyl ester |
SCC-9 oral cancer
cells |
Inhibits MMP-2 expression by up regulation of tissue inhibitor of metalloproteinase-2 (TIMP-2), reducing focal adhesion kinase (FAK) phosphorylation and by the activation of p38/MAPK and JNK |
[91] |
| linoleic acidto α-linolenic acid |
Sprague-Dawley rats and Human chondrocytes |
Inhibits MMP-13 expressions by blocking the IL-1 mediated stimulation |
[92] |
| Nobiletin |
U2OS and
HOS cells |
Down regulates MMP-2 and MMP-9 expressions via ERK and JNK pathways and by inactivating NF-κB, CREB, and SP-1 |
[93] |
| Resveratrol |
RA FLS cells |
Blocks MMP-3 by inhibiting of PI3K-Akt signaling pathway |
[82] |
| Quercetin |
U87-MG glioblastomaand U251 and SHG44 glioma cell lines |
Reduces MMP-9 by blocking the Ras/MAPK/ERK and PI3K/AKT pathways |
[94] |
| Kaempferol |
human tongue squamous cell carcinoma SCC4 cells |
Inhibits MMP-2 expression by inhibiting c-Jun activity and phosphorylation of ERK1/2 |
[84] |
| Naringenin |
human prostate cancer cells |
Reduces the expression of MMPs by inhibiting ERK1/2 and the levels of reactive oxygen species (ROS) |
[95] |
| Diallyl Sulfide, Diallyl Disulfide, and DiallylTrisulfide |
Human Colon
Cancer Cells |
Inhibits MMP by down-regulating the expression of PI3K, Ras, MEKK3, MKK7, ERK1/2, JNK1/2, and p38 |
[96] |
| Epigallocatechin-3-gallate (EGCG) |
RA FLS cells |
Inhibits MMP-1 and MMP-13 by blocking RANTES/CCL5 expression and phosphorylation of JNK p46 |
[97] |
| Luteolin |
A431-IIICancer Cells |
Suppresses MMP secretion by attenuating the phosphorylation of cortactin and Src |
[93] |
| Gallic acid |
human oral cancer cells |
Inhibits MMPs by reducing FAK, MEKK3, PERK, p-p38, p-JNK1/2, p-ERK1/2, SOS1, RhoA, Ras, PKC, p-AKT(Thr308), PI3K, NF-κB p65 |
[83] |
Table 2: Role of various MMP inhibitors on some recent animal and cell culture studies.
Similarly, synthetic molecules have also shown good activity against MMP family. DX-2400, a synthetic component which showed inhibiting activity of MMPs in HT-1080 cells line by attenuating proMMP-2 level [85]. More than 50 chemical compounds are in the clinical trials, although the first clinical trial of MMP inhibitor against cancer was a failure [86]. Many of the MMP inhibitors found not ideal, metabolically unstable, less oral bioavailable and show unwanted side effects. The inhibitory effects of MMP inhibitors were observed well in animal models. However, they were found disappointed when used in small clinical trial due to presence of other diseases in the volunteers. As MMP is basically regulated by TIMP, inhibitors of TIMP can also be a good choice. Previous study found that using TIMP inhibitor (TIMP-1,2,3 and 4) on the catalytic site of MMP might not provide good activity because TIMPs always differ in their affinity for specific MMP family members [86]. However, no drug is hundred percent specific, potent and may often exert side effects. Using proper evaluation, docking, structure activity relationship and large clinical trials might bring a good molecule to treat the diseases like inflammation or cancer.
Limitations of MMP Inhibitors
Understanding the characteristics, structure, domain and function of these key enzymes may have significant applications for several drug therapies like cancer and auto immune diseases. Since last 2 decades, explorations on MMP inhibitors are largely evaluated to introduce a new and effective molecule. Several inhibitor have been applied against MMP proteomase but due to having Zn2+ binding domain, many good molecules failed to achieve their activity [98]. Monoclonal antibody has been also proposed against MMP domain and found quite effective but those proteins were never applied further due to having much supporting data [85].
In case of MMP-2 and MMP-9, they contain fibronectin type II inserts within the catalytic domain. The membrane-type MMPs (MT-MMPs) carry a transmembrane domain at the Cterminal end of the hemopexin-like domain. The hemopoxin domain is absent in MMP7. B represents - basic domain structure of human matrix metalloproteinase 2(MMP2). The image were processed using PyMol(The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.) and UCSF Chimera package. Inspired from [37,38,99] (Figure 4).
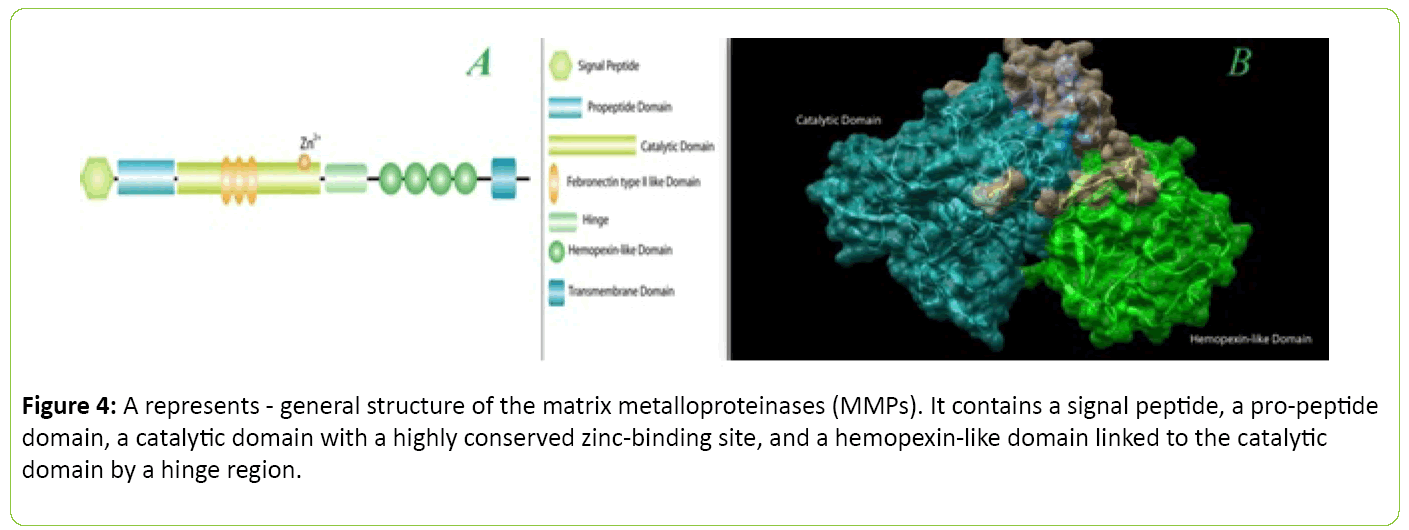
Figure 4: A represents - general structure of the matrix metalloproteinases (MMPs). It contains a signal peptide, a pro-peptide domain, a catalytic domain with a highly conserved zinc-binding site, and a hemopexin-like omain linked to the catalytic domain by a hinge region.
Future Direction and Conclusion
In conclusion, our literature review suggests that the MMPs are playing a significant role in the regulation of many cell types. Most of them are also associated with cellular damage and disease induction. So, measurement of MMPs in various diseases can be used positively to evaluate pathophysiologic condition. Thus, MMP inhibitors can serve as potential drug candidate against many diseases.
18056
References
- Sagor AT, RezaHM, Tabassum N, Sikder B, Ulla A, et al. (2016) Supplementation of rosemary leaves (Rosmarinusofficinalis) powder attenuates oxidative stress, inflammation and fibrosis in carbon tetrachloride (CCl4) treated rats. CurrNutr Food Sci 12: 1-8.
- Sagor MAT, Tabassum N, Potal MA, Alam MA (2015) Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats.Oxid Med Cell Longev2015: 478039.
- Alam MA, ChowdhuryMRH, JainP,Sagor MAT, Reza HM (2015) DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. DiabetolMetabSyndr 7: 1-10.
- Langenskiöld M, Holmdahl L, Falk P, Ivarsson ML (2005) Increased plasma MMP-2protein expression in lymph node-positive patients with colorectal cancer. Int J Colorectal Dis 20: 245-252.
- Katzmarzyk PT, Church TS, Craig CL, Bouchard C(2009) Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998-1005.
- Mohib MM, Hasan I, Chowdry KW, Chowdry Nu, Mohiuddin S, et al. (2016) Role of angiotensin II in hepatic inflammation through MAPK pathway: a review. JHepatitis 2: 1-13.
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, et al. (2003) HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest 112: 160-169.
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, et al. (2000) Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes and intrauterine infection.Am J ObstetGynecol 183: 94-99.
- Kahari VM, Saarialho-Kere U (1997) Matrix metalloproteinases in skin.ExpDermatol 6: 199-213.
- Liu Y, Min D, Bolton T, Nubé V, Twigg SM, et al. (2009) Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes care 32: 117-119.
- Death AK, Fisher EJ, McGrath KC, Yue DK (2003) High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis 168: 263-269.
- Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH (2006) Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. The FASEB journal 20: 1898-1900.
- Yu Q, Stamenkovic I(2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev14: 163-176.
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, et al. (2003) Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease.Circulation 107: 1579-1585.
- Nissinen L, KahariVM (2014) Matrix metalloproteinases in inflammation. Biochimica et BiophysicaActa 1840: 2571-2580.
- Okamoto K, Mimura K, Murawaki Y, Yuasa I ( 2005) Association of functional gene polymorphisms of matrix metalloproteinase (MMP)â€ÂÂÂÃÂ1, MMPâ€ÂÂÂÃÂ3 and MMPâ€ÂÂÂÃÂ9 with the progression of chronic liver disease. J GastroenterolHepatol 20: 1102-1108.
- Zamboni P, Scapoli G, Lanzara V, Izzo M, Fortini P, et al.(2005) Serum iron and matrix metalloproteinaseâ€ÂÂÂÃÂ9 variations in limbs affected by chronic venous disease and venous leg ulcers.DermatolSurg 31: 644-649.
- Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT (1998) Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 78: 1077-1087.
- Asahi M, Wang X, Mori T, Sumii T, Jung JC , et al. (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci21: 7724-7732.
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M , et al.(2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106:55-62.
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, et al. (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis.Nat Cell Biol 2: 737-744.
- Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations.Science 29: 2387-2392.
- Fraser A, Fearon U, Reece R, Emery P, Veale DJ (2001) Matrix metalloproteinase 9, apoptosis, and vascular morphology in early arthritis. Arthritis Rheumatism 44: 2024-2028.
- Ye S (2000) Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix 19: 623-629.
- Bucchieri F, Marino Gammazza A, Pitruzzella A, Fucarino A, Farina F, et al. (2015) Cigarette smoke causes caspase-independent apoptosis of bronchial epithelial cells from asthmatic donors. PloS one 10: e0120510.
- Yang SW, Lim L, Ju S, Song H, Choi DH, et al. (2015) Corrigendum to effects of matrix metalloproteinase 13 on vascular smooth muscle cells migration via Akt–ERK dependent pathway. Tissue and Cell 47: 115-121.
- Kaplan A, Spiller KJ, Towne C, Kanning KC, Choe GT, et al.(2014) Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration.Neuron 81: 333-348.
- Ermolli M, Schumacher M, Lods N, Hammoud M, Marti HP (2003) Differential expression of MMP-2/MMP-9 and potential benefit of an MMP inhibitor in experimental acute kidney allograft rejection. Transplant immunology 11: 137-145.
- Corbel M, Belleguic C, Boichot E, Lagente V (2002) Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell biology and toxicology 18: 51-61.
- Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B (2000) Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization.Exp Eye Res 70: 419-428.
- Gutierrez-Fernandez A, Inada M, Balbın M, Fueyo A, Pitiot AS, et al. (2007) Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8).The FASEB Journal 21: 2580-2591.
- Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm doxycycline selectively depletes aortic wall neutrophils and cytotoxic t cells. Circulation 119: 2209-2216.
- Wojtowicz-Praga SM, Dickson RB, Hawkins MJ(1997) Matrix metalloproteinase inhibitors. Invest New Drugs 15: 949-964.
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, et al. (2001) MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res263: 209-223.
- Löffek S, Schilling O, Franzke CW (2011) Biological role of matrix metalloproteinases: a critical balance. EurRespir J 38: 191-208.
- Nagase H (1996) Activation mechanisms of matrix metalloproteinases.BiolChem 378: 151-160.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera-a visualization system for exploratory research and analysis. J ComputChem 25: 1605-1612.
- Gross J, Lapiere CM (1962) Collagenolytic activity in amphibian tissues: a tissue culture assay. Proceedings of the National Academy of Sciences 48: 1014-1022.
- Lia NG, Shib ZH, Tang YP, Duan JA (2009) Selective matrix metalloproteinase inhibitors for cancer.Curr Med Chem 16: 3805-3827.
- Sagor AT, Chowdhury MR, Tabassum N, Hossain H, Rahman MM, et al. (2015) Supplementation of fresh ucche (Momordicacharantia L. var. muricataWilld) prevented oxidative stress, fibrosis and hepatic damage in CCl treated rats. BMC Complement Altern Med 15: 115.
- Rack A, Belohradsky BH, Grantzow R, WintergerstU, PflugerT, et al. (2016) Inflammatory pseudotumor (IPT)-surgical cure of an inflammatory syndrome. Eur J Pediatr 175: 903
- Hidemi Takeuchi, Haruhito A Uchida, RyokoUmebayashi, Yuki Kakio, Yuka Okuyama, et al. (2015) The effect of fisetin on lipopolysccharide-induced inflammation and mmp activity in mouse peritoneal macrophages. ArteriosclerThrombVascBiol 35: A216.
- Chowdhury MR, Sagor MA, Tabassum N, Potol MA, Hossain, et al.(2015) Supplementation of citrus maxima peel powder prevented oxidative stress, fibrosis, and hepatic damage in carbon tetrachloride (CCl4) treated rats. Evid Based ComplemenAlternat Med 2015:598179.
- Md Abu TaherSagor, Mohib MM,Tabassum N, Ahmed I, Reza Mh, et al. (2016) Fresh seed supplementation of syzygiumcumini attenuated oxidative stress, inflammation, fibrosis, iron overload, hepatic dysfunction and renal injury in acetaminophen induced rats. J Drug MetabToxicol7: 208.
- Hsieh HL, Yang CM (2014) The role of matrix metalloproteinase-9 in pro-inflammatory factors-induced brain inflammation and neurodegenerative diseases. Inflammation Cell Signaling1:e124.
- Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, et al. (2013) Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. JBiolChem288: 598-607.
- Blackburn JS, Brinckerhoff CE (2008) Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am JPatho173:1736-1746.
- Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, et al. (2006) Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease. JPharmacoExpTher318: 741-750.
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, et al. (2000) Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science289: 1202-1206.
- McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, et al. (2001) Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. JBiolChem276:43503-43508.
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, et al.(2002) Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood100: 1160-1167.
- Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. NatRev Immuno4: 617-629.
- Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M (2011) Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology280:152-163.
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, et al. (1997) Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. ProcNatiAcadSci USA 94:7959-7964.
- Reza HM, MAT Sagor,MA Alam (2015) Iron deposition causes oxidative stress, inflammation and fibrosis in carbon tetrachloride-induced liver dysfunction in rats. Bangladesh JPharmac10:152-159.
- Gardi C, Arezzini B, Fortino V, Comporti M (2002) Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. BiochemPharmacol64:1139-1145.
- RammGA,RuddellRG (2005) Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis.Semin Liver Dis 25: 433-449.
- Cipollone F, Fazia M, Iezzi A, Zucchelli M, Pini B, et al. (2003) Suppression of the functionally coupled cyclooxygenase-2/prostaglandin E synthase as a basis of simvastatin-dependent plaque stabilization in humans. Circulation107:1479-1485.
- Renehan AG, C Booth, CS Potten (2001) What is apoptosis, and why is it important? BM J322: 1536-1538.
- Zhang Y, Wang T, Li Y, Zhao P, Huang J, et al. (2016) Abstract TP258: Enoph1-ADI1-MT-MMP-1 pathway contributes to ischemic brain microvascular endothelial cell apoptosis in vivo and in vitro. Stroke47:ATP258.
- Seo HS, Ku JM, Choi HS, Woo JK, Jang BH, et al. (2015)Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol Med Rep12:2977-2984.
- Yang CM, Hsieh HL, Lin CC, Shih RH, Chi PL, et al.(2013) Multiple factors from bradykinin-challenged astrocytes contribute to the neuronal apoptosis: involvement of astroglial ROS, MMP-9, and HO-1/CO system. MolNeurobiol47:1020-1033.
- Williams H, Johnson JL, Jackson CL, White SJ, George SJ (2010) MMP-7 mediates cleavage of N-cadherin and promotes smooth muscle cell apoptosis. Cardiovasc Res 87: 137-146.
- Lee SR, Lo EH (2004) Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia–reoxygenation. JCereb Blood FlowMetab24:720-727.
- Siegel RL, Miller KD,Jemal A (2015) Cancer statistics Cancer.J Clinicians65:5-29.
- Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, et al. (2015) Prediction of long-term other-cause mortality in men with early-stage prostate cancer: results from the Prostate Cancer Outcomes Study. Urology85:92-100.
- Byers T,Sedjo RL (2015) Body fatness as a cause of cancer: epidemiologic clues to biologic mechanisms. Endocr Related Cancer22:R125-R134.
- Freddie Bray, AhmedinJemal, Lindsey A Torre, David Forman,Paolo Vineis (2015) Long-term realism and cost-effectiveness: Primary prevention in combatting cancer and associated inequalities worldwide. J Nati Cancer Inst107: djv273.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010–2020. JNatl Cancer Inst103: 117-128.
- Jin Yu, Xie Q, Zhou H, Peng H, Lu H, et al. (2016)Survivin, MMP-2, and MMP-9 expression in different types of cervical lesions and correlation analysis. Int J ClinExpPathol9:5445-5451.
- Castro-Castro A, Marchesin V, Monteiro P, Lodillinsky C, Rossé C, et al. (2016) Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell invasion. Annu RevCellDevBiol32.
- Schwegmann K, Bettenworth D, Hermann S, Faust A, Poremba C, et al. (2016) Detection of early murine colorectal cancer by MMP-2/-9–guided fluorescence endoscopy. Inflamm Bowel Dis22: 82-91.
- Nguyen HL, Kadam P, Helkin A, Cao K, Wu S, et al. (2016) MT1-MMP activation of TGF-β signaling enables intercellular activation of an epithelial-mesenchymal transition program in cancer. Curr Cancer Drug Targets 16: 618-630.
- Darlix A, Lamy PJ, Lopez-Crapez E, Braccini AL, Firmin N, et al. Serum NSE, MMPâ€ÂÂÂÃÂ9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: predictive biomarkers for brain metastases? IntJ Cancer 139: 2299-2311.
- Vihinen P, Ala-aho R,Kahari VM (2005) Matrix metalloproteinases as therapeutic targets in cancer. CurrCancer Drug Targets5:203-220.
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, et al. (2000) Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. JInvest Dermatol114:480-486.
- Cross JB, Duca JS, Kaminski JJ, Madison VS (2002) The active site of a zinc-dependent metalloproteinase influences the computed pk(a) of ligands coordinated to the catalytic zinc ion. JAmChemSoc124:11004-11007.
- Tu G, Xu W, Huang H, Li S (2008) Progress in the development of matrix metalloproteinase inhibitors. Curr MedChem15:1388-1395.
- Aroui S, Aouey B, Chtourou Y, Meunier AC, Fetoui H, et al. (2016)Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chemico-biological interactions244:195-203.
- Tian J, Chen JW, Gao JS, Li L, Xie X (2013) Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. RheumatolInt33:1829-1835.
- Kuo CL, Lai KC, Ma YS, Weng SW, Lin JP, et al. (2014) Gallic acid inhibits migration and invasion of SCC‑4 human oral cancer cells through actions of NF‑κB, Ras and matrix metalloproteinase-2 and-9. Oncol Rep32:355-361.
- Lin CW, Chen PN, Chen MK, Yang WE, Zang CH, et al.(2013) Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLOS One8:e80883.
- Devy L, Huang L, Naa L, Yanamandra N, Pieters H, et al. (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res69: 1517-1526.
- Overall CM, Lopez Otin C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer2:657-672.
- Yang SW, Lim L, Ju S, Choi DH, Song H (20150 Effects of matrix metalloproteinase 13 on vascular smooth muscle cells migration via Akt–ERK dependent pathway. TissueCell 47:115-121.
- Castro MM, Fuah J, Ali M, Sung M, Schulz J, et al. (2013) Inhibitory effects of caspase inhibitors on the activity of matrix metalloproteinase-2. BiochemPharmacol86:469-475.
- Konerua M, Sahua BD, Kumarb JM, Kunchaa M, Kadaria A, et al. (2016)Fisetin protects liver from binge alcohol-induced toxicity by mechanisms including inhibition of matrix metalloproteinases (MMPs) and oxidative stress. J Functional Foods22:588-601.
- Sani IK, Marashi SH, Kalalinia F (2015)Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. ToxicolVitro 29:893-900.
- Peng CY, Yang HW, Chu YH, Chang YC, Hsieh MJ, et al.(2012) Caffeic Acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway. EvidBased ComplemenAlternat Med 2012:732578.
- Yu H, Li Y, Ma L, Meng H, Bai X, et al. (2015) A low ratio of n-6/n-3 polyunsaturated fatty acids suppresses matrix metalloproteinase 13 expression and reduces adjuvant-induced arthritis in rats. Nutr Res35: 1113-1121.
- Lin YC, Tsai PH, Lin YC, Cheng CH, Lin TH, et al.(2013) Impact of flavonoids on matrix metalloproteinase secretion and invadopodia formation in highly invasive A431-III cancer cells. PloS one 8: 71903.
- Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK, et al.(2015) Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. NeurochemInt80:60-71.
- Lin EJ, Zhang X, Wang Dy, Hong SZ, Li LY (2014) Naringenin modulates the metastasis of human prostate cancer cells by down regulating the matrix metalloproteinases-2/-9 via ROS/ERK1/2 pathways. Bangladesh J Pharm9: 419-427.
- Lai KC, Hsu SC, Kuo CL, Yang JS, Ma CY, et al. (2013) Diallyl sulfide, diallyl disulfide, and diallyltrisulfide inhibit migration and invasion in human colon cancer colo 205 cells through the inhibition of matrix metalloproteinaseâ€ÂÂÂÃÂ2,â€ÂÂÂÃÂ7, andâ€ÂÂÂÃÂ9 expressions. EnvironToxicol28: 479-488.
- Agere S, NahidAkhtar, SharayahRiegsecker,Salahuddin Ahmed (2015) Epigallocatechin-3-gallate (EGCG) inhibits RANTES/CCL5 induced MMP-1 and MMP-13 expression in human rheumatoid arthritis synovial fibroblasts. FASEB J 29: LB280.
- Cathcart J,PulkoskiGross A, CaoJ (2015) Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes & Diseases2: 26-34.
- Massova I, Kotra LP, Fridman R, Mobashery S (1998) Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 12: 1075-1095.









