Keywords
|
| Melanoma cells, Anomalous cancer cells, Photon emissions, Ultraweak luminescence, Mice, Tumors |
Introduction
|
| The physical-chemical characteristics of tumors are expected to reflect both elemental and aggregate properties. Elemental properties are those that define the single cell while aggregate features would reflect emergent properties because of that aggregate. From the seminal works of Gurwitch [1] and more recently Popp [2] there is clear evidence that all living systems emit photons as ultraweak light that can be measured by photomultiplier units (PMTs). The flux densities of the photon emissions are in the order of 10-12 to 10-11 W·m-2 [3,4]. Van Wijk and Aken [5] have contributed significantly to the understanding of photon emissions in the general area of tumor biology. We [6] have shown that several lines of malignant cells emit copious photons when they are removed from standard incubation conditions (37°C) and maintained at room temperature. The shift in the photon frequencies, from infrared to ultraviolet, occurs over durations of several hours and is associated with specific increases in activity of portions of classic molecular signaling pathways in the cell [7]. |
| The contemporary assumption is that the plasma membranes of cells (including cancer cells) from experimental cell lines dissipate within 24 hr after removal from optimal incubation. However we have shown there is minimal evidence that the integrity of the membrane is eliminated as inferred by staining with trypan blue [6]. In addition Dotta et al. [8] found that cells in dishes that no longer emitted photons discernable by PMTs would exhibit “reactivation” of photon emissions if there was increased global geomagnetic activity above about 40 nT during a period up to 3 days after the removal from incubation and nutrition. While examining methods to eliminate the growth of injected B16-BL6 mouse melanoma cells into C57 mice we measured an interesting property of these cells both as suspensions from the original culture and after they had been removed as complete tumors from the mice about 3 to 4 wk later. |
Materials and Methods
|
| In two separate experiments a total 24 (2 to 4 per cage) C57 male mice (100 to 150 days of age) housed in standard cages and light: dark environments were recruited as an ancillary component to the central study which has involved hundreds of male C57 mice [9]. In the latter study mice were injected subcutaneously in the right flank with 100 μL of a solution of (0.5 to 1 million) B16-BL6 melanoma cells from a Hamilton syringe within ~1 hr after they had been prepared according to the approved animal protocol. We considered this large sample to be an appropriate reference for any unusual features that might emerge following our experimental manipulation. |
| For the present study the vials of 10 to 15 cc suspensions of melanoma cells (total of ~108 cells) remained at room temperature for 3 days within the colony room. One, 2, and 3 days later 100 uL were extracted from a vial and injected in the same manner (subcutaneously) in the right flank of each of the 24 mice (8 mice per group). By day two the smell of the suspension of cells was conspicuously fetid. After the injection the mice were not disturbed and were attended by the animal care technicians. The health of the mice was monitored along with all of the other mice that had received the cells within 1 hr after preparation. When the tumors emerged and reached the clinical criteria for termination all mice were killed by carbon dioxide or decapitation |
| The tumors from the delayed melanoma injections grew to typical sizes. They were removed on criteria days determined by the animal care protocol by excising the skin and cutting around the tumor before it was placed on weighing paper for measurement. The characteristics of the tumor were examined by gross dissection and compared to the typical melanoma. They were fixed in ethanol-formalin-acetic acid, processed, sectioned by a microtome (6 μm) and stained with toluidine blue O. |
| For the PMT measurements the typical melanomas (those that grew from subcutaneous injections within an hour after preparation) were placed in plastic (covered) culture cell dishes. The bottoms of the dishes were placed over the aperture (12.6 cm2) of a PMT sensor that was housed in a black box covered with several layers of black terry cloth towels such that background approached an asymptote around 10-12 W·m-2. The measurements were taken once per min continually (1440 measurements per day). The box was kept in a dark room in the basement of a building on the university campus. An increase of 1 unit (of scale between 1 and 100) reflected an increased of 5·10-11 W·m-2. Additional details for the equipment and calibrations have been described elsewhere [8,10]. Measurements of photons flux density were obtained every minute over the subsequent three days. |
Results
|
| The results were conspicuous. For the last approximately 300 mice injected over the last few years with 100 μL of 0.5 to 1 million cells every mouse developed conspicuous melanoma tumors over the injection sites within 3 to 4 wk. The percentages of mice that developed similar sized tumors over the injection site that received the melanoma cells that sat at room temperature in open air for 1, 2, or 3 days were 100%, 62%, and 50%, respectively. The termination dates of these mice based upon the sizes of the tumors were within the range of those associated with mice injected within an hour of preparation. When the tumors were removed and dissected they were qualitatively different from the typical melanoma tumor. Although they were encapsulated and vascularized, they were less viscous. The olfactory stimuli were remarkable fetid and indistinguishable from the smell of the vial from which the cells were obtained for injection. |
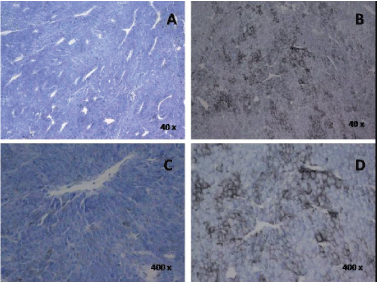
| The histopathology was also qualitatively different. As noted in Figure 1, these cells (B, D) did not exhibit the deep basophilic stain within the cytoplasm of the proliferating B16 cells. There was also marked increase in melanin production. The histology of the typical melanoma tumor in Figure 1A and 1C was what we have observed in sections of dozens of other similar tumors. Note that the presence of reduced vascularization (open spaces) in B and D. In other words the cells that remained at room temperature in the open air for three days still proliferated but without the apparent density of blood vessels that accompanies typical melanoma tumors. |
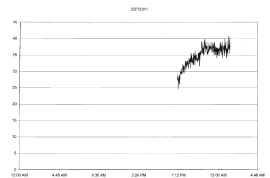
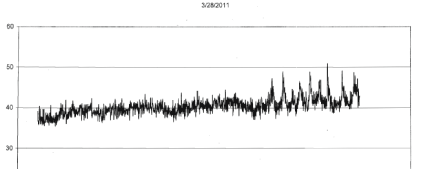
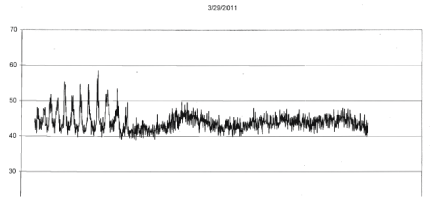
| For the four different runs with tumors (1 to 2 gm) extracted from the mice that had been injected with the same B16 cells within an hour after removal from incubation the photon emissions were also qualitatively obvious. Within the first 3 to 4 hr after removal from the mice, the photon flux densities increased from the background around 10-13 W·m-2 to 5·10-11 W·m-2. An example of the most obvious effect is shown in Figure 2. In addition, during the subsequent three days there were occasional bursts of photon emissions. The most distinctive example, shown in Figures 3-5 (same mouse) revealed enhanced photon emissions with a periodicity of about 40 min that was maintained for about 14 hrs. During the peak photon flux densities the values approached 6.5·10-10 W·m-2. After day 4 (data not shown) the photon emissions slowly attenuated. |
Discussion
|
| The vitality of cancer cells removed from classical environments such as the incubator or tumor has been assumed to be limited. This is considered relevant for both radiation and chemical treatments. Although a small percentage of malignant cells often survive and their resilient properties are assumed to be responsible for the subsequent “treatment resistance”, a form of habituation or “tolerance” to the previous stimuli, or “natural selection” there may be other mechanisms involved with posttreatment recurrence or proliferation at distal sites. If these anomalous cells with different histological features occurred within a clinical context, they could alter the characteristics of the tumor. |
| If the microenvironment of the central portion of tumors is comparable to the conditions of allowing suspensions of cells to be exposed to ambient temperature and air rather than a specific temperature and gas mixture within the incubator, the results of this study indicated that the capacity for most of the melanoma cells to be reactivated is significant. Moreover the proliferations of those tumors appear to express the characteristics of those source cells that remained at room temperature exposed to air. The diminished staining, if it is a valid indicator of the intracellular chemistry, would indicate there is minimal cytoplasmic involvement with this proliferation. This could have significant clinical implication for treatments based upon direct involvement with processes within the cytoplasm. |
| The signal-like, periodic emissions of photons from tumor masses more than 48 hrs after removal of blood supply and oxygen, when necrosis and dissipation of the integrity of cells membranes are supposed to be occurring, has potential application for metastases. Injections of morphine (a substance known to sometimes promote metastases of tumors) into cell cultures maintained in standard incubation produce significant “burst” emissions of photons for several hours [11]. If photons are the primary mode of communication between cells (as suggested by Trushin [12], Fels [13], and Bajpai et al. [14]), then the “information patterns within photon emissions from dying tumors from spontaneous sources or treatment intervention may explain the later re-occurrence of the tumors or their proliferation elsewhere in the body. |
| During the three days after the removal of the tumor from the mouse the photon flux density emitted by the 1 gm mass increased from ~0.6·10-12 W·m-2 to about 5·10-11 W·m-2 within about 3 hr. With an aperture of 1.26 ·10-3 m2 this would be equivalent to ~6·10-15 J per s. Assuming the photons were emitted in all directions from the 1 gm tumor the actual photon emission would have been about 6 times greater or ~4·10-14 J per s. If there are ~108 cells per gm of tumor as calculated by Del Monte [15], the energy per cell would be 10-22 to 10-21 J per s. However the peak emissions in flux power densities during the approximately 8 hrs of the more synchronous ~40 min oscillations were about a factor of 10 higher, or about 10-21 to 10-20 J per s per tumor cell within the mass. This continued despite the absence of blood flow or normal oxygenation. The later increment of energy (~10- 20 J) is involved with a multitude of plasma membrane and receptor-agonist phenomena [16]. |
| Dotta and Persinger [17,18] have shown experimentally and by quantification that the lateral diffusion of charged components through and around the plasma membrane displays a “membrane magnetic moment” that when exposed to a very specific weak intensity and temporally patterned magnetic field can enhance photon emissions from aggregates of melanoma cells. Simulation of these conditions experimentally by “rotating” magnetic fields with changing angular velocities in different loci resulted in strong evidence of non-locality. In other words there was excess correlation of photon emission patterns between spaces separated by non-traditional distances if they shared the same temporal shifts in circular (rotating) magnetic fields with changing angular velocities. This effect may offer other explanations for the phenomenon of “metastasis” that could involve “non-local” effects which would not require transport through either blood supply or cellular migration. |
Acknowledgement
|
| Thanks to Dr. Blake T. Dotta, Dr. Linda S. St-Pierre, and Dr. Robert M. Lafrenie for technical contributions. |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
| |










