Tomasz Siwek1*, Monika Barczewska1, Lukasz Grabarczyk1, Mariusz Sowa1, Katarzyna Jezierska-Wozniak1,3, Aleksandra Habich1,3, Joanna Wojtkiewicz2,3, Wanda Badowska4 and Wojciech Maksymowicz1,
1Department of Neurology and Neurosurgery, School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Poland
2Department of Pathophysiology, School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Poland
3Laboratory for Regenerative Medicine School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Poland
4Department of Clinical Pediatrics, School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Poland
- *Corresponding Author:
- Tomasz Siwek
MD, Department of Neurology and Neurosurgery
School of Medicine, Collegium Medicum
University of Warmia and Mazury in Olsztyn
10-082 Olsztyn ul. Warszawska 30, Poland
Tel: +48 895245347
E-mail: tomasz.siwek@uwm.edu.pl
Received date: May 14, 2018; Accepted date: June 11, 2018; Published date: June 14, 2018
Citation: Siwek T, Barczewska M, Grabarczyk L, Sowa M, Jezierska-Wozniak K, et al. (2018) Mesenchymal Stem Cell (MSC) Transplantation in Patients with Amyotrophic Lateral Sclerosis (ALS): Is there a “Responder Population”?. J Neurol Neurosci Vol.9 No.3:260. doi: 10.21767/2171-6625.1000260
Keywords
Mesenchymal stem cells; Bone marrow; Amyotrophic lateral sclerosis (ALS); Stem cell therapy; Responders to treatment
Introduction
Amyotrophic lateral sclerosis is a disease which still remains untreatable [1,2]. It is usually fatal disease of the upper and lower motoneuron. More than 20 years after approval of riluzole, no new drugs have shown proven efficacy. Hence, there is a clear need for new therapeutic approaches [3]. In recent years potential benefits of stem cell-based approaches have been demonstrated making stem cells an interesting candidate for new ALS therapy [4]. Although replacing motoneurons does not seem to be as promising as initially expected, there is a growing interest in targeting the environment of motoneurons (i.e., microglia and astrocytes). Among many types of stem cells human embryonic cells are definitely the most powerful type [5]. However, mesenchymal stem cells (MSCs) have several attributes that make them good candidates for cell-based therapies too [6]. Firstly, by using MSCs we can avoid the ethical issues of embryonic or fetal derived stem cells. Moreover, MSCs are not immunogenic, they are easy to derive and provide the possibility of autologus transplantation [7]. In several recent pre-clinical and clinical trials MSCs-based approaches have been shown to be safe and feasible.
Objectives and Methods
To evaluate the feasibility, safety, and possible clinical effects of intrathecal administration of autologous mesenchymal stem cells (MSCs) in patients with amyotrophic lateral sclerosis (ALS) [8]. The design has been approved by the Ethic Committee of University of Warmia and Mazury (UWM) in Olsztyn, Poland. Written informed consent was obtained prior to the inclusion of the study from each participant. The trial has been registered under NCT02881489.
Patients between the ages of 18 and 65 years with definite sporadic ALS according to the El Escorial Revised Criteria (17) were eligible for the study. As of 01.09.2014, 30 patients (20 males and 10 females) have been consecutively enrolled and transplanted with MSCs. The mean age at enrolment was 49.5 (± 12.85) years. The mean ALSFRS-R at enrolment was 32 points and the mean (FVC) 72%. There were 6 patients presenting with bulbar signs at enrolment. The follow-up is ongoing and planned for 36 months after the cell-based treatment. Out of the group of 30 patients the data of 25 patients (who had at least 3 examinations post-transplant) have been available for the interim analysis 6 months after the treatment. Three patients died during the observation period and were lost to follow-up. Two patients have been excluded from the analysis by the study steering committee: one patient developed a subdural hematoma after falling down the stairs and showed- despite neurosurgical intervention- a consecutive severe neurological deficit not related to the ALS. Another patient developed severe aspiration pneumonia 3 months after stem cells therapy and required mechanical ventilation in a critical care unit. The study steering committee did not consider those complications as adverse effects of the transplant procedure.
Approximately 200 mL of bone marrow was obtained from each patient in local anesthesia from the posterior iliac crest. A culture of purified MSCs was prepared under aseptic GMP conditions by the European Medicines Agency in 1999, where manufacturing facilities maintain a clean and hygienic manufacturing area, in controlled environmental conditions. All manufacturing processes are clearly defined, controlled and validated to ensure consistency and compliance with specifications. The laboratory has all the approvals and certificates required by Polish and European law.
Mesenchymal cells were cultured until they reached confluency, then harvested and passaged (no longer than to 30 days in culture and 2 passages) [9]. A sample of the cells to be injected was tested by fluorescence-activated cell sorter (BD FACSAria II) analysis for the presence of the surface markers characteristic for MSCs (CD73, CD90, CD105,) according to MSC features established by the International Society for Cellular Therapy guidelines [10]. The same procedure was used for all patients, which allowed for the establishment of the reproducible product to warrant the series of quality controls required to certify the safety, identity, potency and the pharmaceutical grade of the MSCs, to satisfy the GMP regulatory process criteria.
Bone marrow collection occurred five weeks before planned administration of mesenchymal stem cells, after 6 months of clinical observation. Before implantation, the cells were maintained for at least 3 hours in basal MSC medium without serum, detached and washed 3 times with PBS 1x containing 1% human albumin and once with autologous cerebrospinal fluid. The cells were suspended in 1 mL of autologous CSF in all patients. The number of cells was determined by analysis in a Burker chamber with Trypan blue staining. A mean of 15×106 cells was injected intrathecally (into cervical, thoracic or lumbar region depending on the clinical symptoms) by neurosurgical procedure using local anesthesia by the same neurosurgeon. A spinal MRI was conducted during the first post-treatment week to exclude structural changes. For outcome measurement a mean rate of change in ALSFRS-R score pre- and post-treatment has been used. This measure shows a linear progressive decline during the course of the disease and is commonly used in clinical trials [11-16]. In order to estimate the individual disease progression rate for each study participant before transplantation, the patients had a six-month period of natural history observation [5]. Each patient was examined by the same study physician every two months. After MSC transplantation patients are to be monitored for at least 36 months by the above clinical assessment performed by the same examiners. First data analysis was planned 6 months after treatment. Patients unable to attend the monitoring center have been contacted by telephone and delivered ALSFRS-R scale and an interview was conducted with them. For every patient a pre- and posttransplant mean rate of ALSFRS-R score change per 2-month period has been calculated. For comparison of measured parameters, nonparametric tests (test U Mann Whitney for unpaired samples and Wilcoxon test for paired samples) were used due to an abnormal distribution of measurement levels.
Results
Safety
There were no side effects after bone marrow collection. However, for technical reasons in one patient the bone marrow aspiration had to be conducted twice. No immediate surgical complications have been observed after the cells-CSF suspension was injected: one patient developed post-dural puncture headache (PDPH). Moreover, no major adverse effects of both bone marrow collection and surgical procedure were reported in any of the patients during a follow-up of up to 6 months. The three deaths were not related to the surgical procedure itself but were a result of the disease progression. No structural changes of the spinal cord or signs of abnormal cell proliferation were detected in the short term in the postsurgery MRI.
Clinical effects
In the six-month post-transplantation period, there was a significant change in the mean rate of clinical progression (ALSFRS-R score) as compared to the 6 months preceding treatment. The mean rate of ALSFRS-R score change (decrease) pre-transplant was 1.76 ± 1.36 points/period whereas the mean post-transplant rate was 1.06 ± 0.9 points/period. This difference reached the statistical significance in Wilcoxon test (p=0.014) (Figure 1).
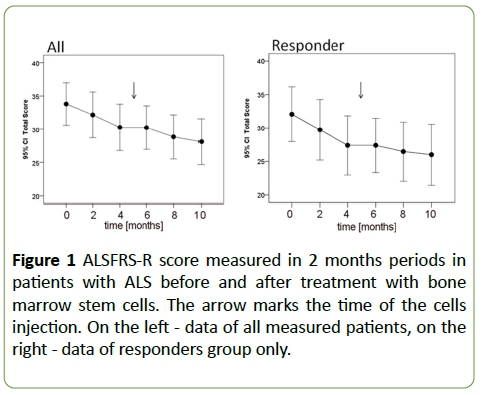
Figure 1: ALSFRS-R score measured in 2 months periods in patients with ALS before and after treatment with bone marrow stem cells. The arrow marks the time of the cells injection. On the left - data of all measured patients, on the right - data of responders group only.
“Responders” vs. “Non-responders”
Interestingly, within the entire group of 25 patients who had received the intrathecal treatment with MSCs and had been analyzed 6 months after transplant, there were patients who seemed to deteriorate slower than the others. Those patients who seemingly deteriorated slower we called “responders” (n=15, nine males and six females).
We defined “responders” as those, whose mean changes ALSFRS-R score in 3 visits before MSCs transplantation, was higher, than the mean of ALS-FRS score in 3 visits after this procedure. All those patients who did not meet the above criteria were called “non-responders” (n=10). For both of those arbitrary distinguished groups (“responders” and “nonresponders”) we performed a post-hoc analysis of the clinical progression pre- and post-transplant. The pre-treatment rate of ALSFRS-R score change per 2-month period in the “responders” group was 2.33 ± 1.48 points/period. The posttreatment rate of change was 0.70 ± 0.94 points/period reaching a significance level of p=0.001 (Figure 2).
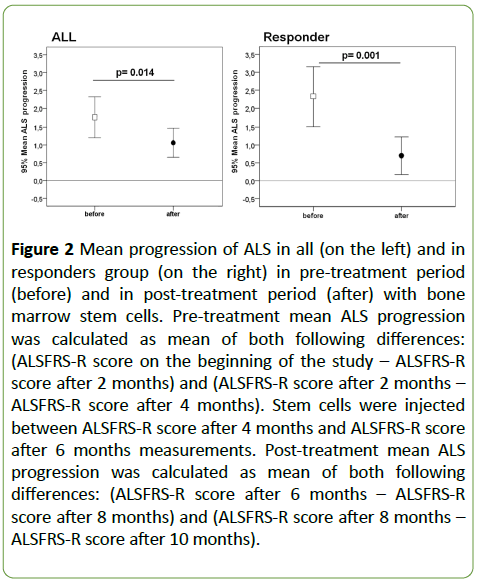
Figure 2: Mean progression of ALS in all (on the left) and in responders group (on the right) in pre-treatment period (before) and in post-treatment period (after) with bone marrow stem cells. Pre-treatment mean ALS progression was calculated as mean of both following differences: (ALSFRS-R score on the beginning of the study – ALSFRS-R score after 2 months) and (ALSFRS-R score after 2 months – ALSFRS-R score after 4 months). Stem cells were injected between ALSFRS-R score after 4 months and ALSFRS-R score after 6 months measurements. Post-treatment mean ALS progression was calculated as mean of both following differences: (ALSFRS-R score after 6 months – ALSFRS-R score after 8 months) and (ALSFRS-R score after 8 months – ALSFRS-R score after 10 months).
In the “non-responders” group the pre-treatment score change was 0.90 ± 0.39 and 1.60 ± 0.84 post-treatment respectively (p=0.016). We have been interested in identifying possible clinical and demographic differences between the “responders” and “non-responders” at the time of enrolment. We found that “responders” had lower ALSFRS total score at enrolment (29.73 ± 7.72 vs. 35.60 ± 7.64, p=0.09) and showed higher pre-treatment rate of progression as compared to “nonresponders” (2.33 ± 1.48 points/period vs. 0.90 ± 0.39 points/ period, p=0.023). “Responders” were somewhat younger than the “non-responders” (mean age 46.53 ± 14.29 vs. 50.08 ± 12.25; p=0.46). Within the “responders” group there were 3 out of 15 patients with bulbar signs at enrolment. There were no differences between both groups in terms of site of spinal injection or injected stem cells number (p=0.14).
Discussion
In recent years number of studies in animal models of ALS have investigated the therapeutic potential of MSCs administered either peripherally or injected directly into the spinal cord. Mazzini et al. performed in 2003 some of the world's first clinical studies to determine the safety and tolerability of direct intraparenchymal transplantation of MSCs to treat ALS. Although they did not show clinical effect of the therapy, no side effects were reported. In a series of follow up studies [17,18] no signs of toxicity or abnormal cell growth were detected, and it was suggested that the treatment might have benefited four patients. In 2010 a second Phase I clinical trial was conducted by Mazzini and her team with expanded patient numbers (n=20) using the same methods as described in the original trial. Again, the treatment has been shown to be safe and feasible although the disease progression in the majority of patients did not appear to be slowed by the transplant.
In addition, in 2010, a Phase I/II open-safety clinical trial by Karussis showed that intrathecal and intravenous administration of autologous bone marrow- derived MSCs into ALS patients is feasible and safe. In this study, patients with ALS or multiple sclerosis were treated either via a standard lumbar puncture (~55 × 106, ~63 × 106 MSCs, respectively) or intravenously (~24 × 106) with MSCs. A more recent Phase I/II clinical trial by the Karussis group and BrainStorm Cellular Therapeutics evaluated the safety, tolerability and therapeutic effects of transplanting MSC-NTF cells into 12 ALS patients at early stages of the disease [19].
Studies by Ki-Wook-Oh in 2015 of repeated intrathecal delivery of BM-MSCs confirm the decline and stabilization of ALS-FRS after treatment. The Staff’s and other’s study from 2016 evaluated the safety of intrathecal autologous adiposederived MSc. It was not designed to assess efficacy; however, no participants had worsened significantly (in assessing the reduction of ALS-FRS), some reported a transient subjective improvement of the clinical condition [20] note in their trial with intrathecal transplantation of BM-MSCs in ALS patients, reduction in ALSFRS decline at 3 months after application (p<0.02) that, in some cases, persisted for 6 months (p<0.05); a better effect was observed in patients in whom the decrease in ALS FRS scores was higher before treatment.
In our phase I trial, 30 intrathecal MSCs transplantation surgeries in 30 ALS subjects were performed. A group of 25 patients was available for the interim analysis 6 months after cell-transplant. Hypothesizing that the areas of tissue damage are widespread throughout the spinal cord, we decided to use the intrathecal approach with different injection sites (cervical, thoracic or lumbar) for stem cell-transplantation to increase the possibility of migration of the injected cells to the proximity of the lesions. Based on our interim analysis 6 months after the treatment, intrathecal administration of autologous bone marrow- derived MSCs into ALS patients is feasible and safe as previously reported. None of our patients experienced significant adverse effects during the 6-month observation period. This is consistent with most previous studies concerning ALS patients treated intraspinally or intrathecally with MSCs [12,13,17,18,21-23] (Table 1).
| Ordinal number |
Patients ID |
Age |
Gender |
ALS form
(Bulbar/Limb) |
Time from first symptoms of ALS
to MSCs transplantation
( In months) |
Groups
(Responders/Non-responders) |
FVC
(%) |
| 1 |
AB |
46 |
M |
L |
26 |
NR |
89 |
| 2 |
JN |
66 |
M |
B |
33 |
NR |
66 |
| 3 |
JC |
56 |
F |
L |
26 |
NR |
111 |
| 4 |
JC |
67 |
M |
B |
15 |
NR |
73 |
| 5 |
TP |
55 |
M |
L |
23 |
NR |
98 |
| 6 |
RP |
45 |
M |
L |
36 |
NR |
75 |
| 7 |
MW |
32 |
M |
L |
28 |
NR |
103 |
| 8 |
JK |
58 |
F |
B |
42 |
NR |
104 |
| 9 |
DJ |
34 |
M |
L |
40 |
NR |
73 |
| 10 |
KC |
53 |
M |
L |
40 |
NR |
101 |
| 11 |
LG |
64 |
M |
L |
30 |
R |
90 |
| 12 |
CT |
56 |
M |
L |
31 |
R |
93 |
| 13 |
AB |
44 |
F |
B |
39 |
R |
75 |
| 14 |
JW |
58 |
M |
L |
20 |
R |
92 |
| 15 |
MS |
37 |
M |
L |
17 |
R |
92 |
| 16 |
JN-J |
32 |
F |
L |
34 |
R |
97 |
| 17 |
AM |
55 |
F |
L |
18 |
R |
92 |
| 18 |
JS |
62 |
F |
B |
37 |
R |
52 |
| 19 |
RZ |
33 |
M |
L |
24 |
R |
82 |
| 20 |
MP |
26 |
F |
L |
46 |
R |
88 |
| 21 |
LL |
32 |
M |
L |
43 |
R |
96 |
| 22 |
MD |
27 |
M |
L |
16 |
R |
67 |
| 23 |
GM |
54 |
M |
L |
25 |
R |
105 |
| 24 |
JN |
68 |
M |
L |
17 |
R |
75 |
| 25 |
AZ |
59 |
M |
L |
31 |
R |
93 |
Table 1: Demographic data, ALS form, duration of the disease from the first symptoms to the experimental procedure, membership of the Responders and Non-responders groups and the value of FVC in recruitment for each participant of the study.
While short term clinical benefits were evident for the entire group of patients the key finding of our study is that there seems to be a group of patients, we call “responders” whose reaction to the treatment was different than the reaction of other patients we call “non-responders”. The difference is that “responders” showed a strong decrease in a mean rate of change in ALSFRS-R score following intrathecal treatment with MSCs. In contrast, in the group of “nonresponders” there seems to be no beneficial effect of the stem-cell treatment (Table 2).
| Variables |
Responders |
Non-responders |
p Test U
Mann-Whitney |
| Mean |
SD |
mean |
SD |
| Patients mean age |
46.53 |
14.29 |
50.80 |
12.25 |
0.46 |
ALSFRS-R – Total score
at enrolment |
29.73 |
7.72 |
35.60 |
7.64 |
0.09 |
| Pretreatment mean ALS progression |
2.33 |
1.48 |
0.90 |
0.39 |
0.002 |
Pre-treatment mean ALS progression was calculated as mean of following differences: (ALSFRS-R score on the beginning of the study – ALSFRS-R score after 2 months) and (ALSFRS-R score after 2 months – ALSFRS-R score after 4 months) Stem cells were injected between ALSFRS-R score after 4 months and ALSFRS-R score after 6 months measurements. SD: Standard Deviation.
Table 2: Patients age and ALS progression in responders and non-responders before stem cells injection.
In our opinion, the identification of those patients who may potentially benefit from cell-based treatment approaches in a prospective manner may be an important tool for classifying ALS patients to cell transplantation procedures. In our study the “responders” were clinically less affected (as measured using ALSFRS) but progressed faster prior to the treatment than “non-responders”. Hence, we hypothesize that the pretreatment progression rate may play a role as a predictive factor and a criterion for selecting ALS patients for cell-based therapies.
Conclusion
In summary, the preliminary results of our interim 6 months’ post-transplantation analysis raise the possibility that intrathecal stem cell transplantation could slow disease progression in a certain subpopulation of ALS patients. This observation is in line with numerous previous studies [12,13,17,18,21-23].
The most important potential limitations of our study are the small sample size, the variability of the disease in selected patients, and lack of a control group.
We agree that prior to clinical translation for ALS; scientific evidence must support the ability of the proposed treatment [24]. On the other hand, new therapeutic approaches are desperately needed to uncover effective treatments for this still untreatable disease. However, in our opinion, based on the presented safety data of stem cell-based approaches for ALS, it is time to implement these therapies in practice to understand whether they may be helpful for our patients [25].
Acknowledgements
The authors would like to thank A. Tutas, M. Sobieszczanska, D. Glod, M. Olchowska-Sobczak, and I. Galewska-Gurzinska for their excellent technical support.
This work was supported by the National Centre for Research and Development (No. STRATEGMED/1/234261/2/2014; JW; WM).
22903
References
- Armon C, Graves MC, Moses D, Forté DK, Sepulveda L, et al. (2000) Linear estimates of disease progression predict survival in patients with amyotrophic lateral sclerosis. Muscle Nerve 23: 874-882.
- Brooks BR (1994) El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 124: 96-107.
- Czarzasta J, Habich A, Siwek T, Czaplinski A, Maksymowicz W, et al. (2017) Stem cells for ALS: An overview of possible therapeutic approaches. Int J Devl Neuroscience 57: 46-55.
- Brinkmann JR, Andres P, Mendoza M, Sanjak M (1997) Guidelines for the use and performance of quantitative outcome measures in ALS clinical trials. J Neurol Sci 147: 97-111.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10): 3838–3843.
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4): 1815-1822.
- English K, Wood KJ (2013) Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med 3(5): 155-160.
- Czaplinski A, Yen AA, Simpson EP, Appel SH (2006) Predictability of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 34(6): 702-708.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 8(4): 315–317.
- Gordon P, Corcia P, Meininger V (2013) New therapy options for amyotrophic lateral sclerosis. Expert Opin. Pharmacother 14(14): 1907-1917.
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, et al. (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5(6): 933-946.
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, et al. (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67(10): 1187–1194.
- Oh KW,Moon C,Kim HY, Oh SI,Park J, et al. (2015) Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med 4(6): 590-597.
- Le Blanc K (2003) Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 5(6): 485–489.
- Lindvall O, Barker RA, Brustle O, Isacson O, Svendsen CN (2012) Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell 10(2): 151–155.
- Magnus T, Beck M, Giess R, Puls I, Naumann M, et al. (2002) Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle Nerve 25: 709-714.
- Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, et al. (2006) Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res 28: 523-526.
- Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, et al. (2008) Stem cell treatment in amyotrophic lateral sclerosis. J Neurol Sci 265(1-2): 78-83.
- Svendsen CN (2013) Back to the future: How human induced pluripotent stem cells will transform regenerative medicine. Hum Mol Genet 22(R1): R32–R38.
- Syková E, Rychmach P, Drahorádová I, Konrádová Š, RuÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂæÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂþicková K, et al. (2017) Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: Results of phase I/IIa clinical trial. Cell Transplant 26(4): 647–658.
- Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, et al. (2003) Stem cell therapy in amyotrophic lateral sclerosis: A methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disorder 4(3): 158-161.
- Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, et al. (2010) Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol 223(1): 229-237.
- Staff NP, Madigan NN, Morris J, Jentoft M, Sorenson EJ, et al. (2016) Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 87(21): 2230-2234.
- Stagg J, Galipeau J (2013) Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med 13(5): 856–867.
- Thomsen GM, Gowing G, Svendsen S, Svendsen CN (2014) The past, present and future of stem cell clinical trials for ALS. Exp Neurol 262: 127-137.







