Nataraj KS*, Prasanna Lakshmi B and Sravanthi I
Department of Pharmaceutical Analysis and Quality Assurance, Shri Vishnu College of Pharmacy, Kovvada, Andhra Pradesh, India
*Corresponding Author:
Nataraj KS
Department of Pharmaceutical Analysis and Quality Assurance, Shri Vishnu College of Pharmacy Kovvada, Andhra Pradesh, India
Tel: +919866250050
E-mail: drnatraj@svcp.edu.in
Received date: July 29, 2017; Accepted date: August 30, 2017; Published date: Setember 01, 2017
Citation: Nataraj KS, Lakshmi PB, Sravanthi I (2017) Method Validation and Estimation of Anagrelide Hydrochloride in Pharmaceutical Dosage by RPHPLC Method. Int J Drug Dev & Res 9: 25-27
Keywords
Anagrelide hydrochloride; RP-HPLC; ICH guidelines; Validation
Introduction
Anagrelide Hydrochloride [1] Monohydrate 6,7-dichloro- 1H,2H,3H,5H-imidazolidino[2,1-b]quinazolin-2-one C10H7Cl2N3O. HCl.H2O Anagrelide HCl [2,3] inhibits phosphodiesterase III which is found in thrombocytes and cause that few analytical methods available for determination of anagrelide [4-8] by RP-HPLC, LC-MS, UV spectrophotometry. The aim of the present work was the development of a RP-HPLC method for the estimation of Anagrelide Hydrochloride in a pharmaceutical dosage for and its validation according to ICH guideline raise in cAMP levels that in turn show the inhibitory effect on platelet aggregation. Two major metabolites, active and inactive, have been identified. The active metabolite, 3-hydroxy Anagrelide, shows similar potency and efficacy as Anagrelide. The inactive metabolite, 5, 6-dichloro-3,4-dihydroquinazolin-2-ylamine, does not participate in the overall effect of Anagrelide. Literature survey revealed.
Materials and Methods
Chromatographic conditions
The present work was aimed at the analytical method development and validation for the estimation of Anagrelide Hydrochloride in capsule dosage form by RP-HPLC method. The chromatographic mode used in this method was RP-HPLC and the detector used in this was PDA detector. Inertsil ODS -3V C18 (150 × 4.6 mm, 5 μ) was used as stationary phase and the mobile phase used in this method was 0.1% triethylamine Milli-Q water, pH 3.0 and Acetonitrile. Ratio of mobile phase used in this was (70:30% v/v). The wave length used for detection was 254.0 nm and flow rate was maintained at 1.0 ml/min and the injection volume was 20.0 μL and the temperature of the column was maintained at 25°C.
Preparation of 0.1% v/v triethylamine buffer, pH 3.0
An accurately measured 1000 mL of Milli-Q water, 1.0 mL of tri ethylamine was added to it and mixed well. The pH of the solution was adjusted to 3.00 ± 0.05 with orthophosphoric acid and filtered through 0.45 μ Membrane filter.
Preparation of standard solution
An accurately weighed amount of 25.0 mg of Anagrelide Hydrochloride working standard was transferred into 100 mL volumetric flask, about 70.0 mL of acetonitrile was added and sonicated for 5 minutes to dissolve and 3 drops of 0.1 M hydrochloric acid were added and the volume was made up with acetonitrile and the contents were mixed well. Diluted 2.0 mL of the above solution into 50 mL volumetric flask with diluent and mixed well (8.2 μg/mL).
Preparation of test solution
An accurately weighed 5 capsules of Anagrelide Hydrochloride were directly transferred into 250 mL volumetric flask, 150.0 mL of diluent was added to it. Sonicated for 30 minutes with intermediate shaking. Maintained the sonicate bath temperature below 25°C throughout the sonication. After sonication, the volume was made up to the mark with diluent and centrifuged the solution at 4000 RPM for 10 minutes. Collected the supernatant and injected into HPLC system.
Results and Discussion
The present work was aimed at the analytical method development and validation for the estimation of Anagrelide Hydrochloride in capsule dosage form by RP-HPLC method.
Accuracy
Accuracy of the method was performed with concentration level of 50%, 100% and 150% and the percentage recovery was calculated and found to be between 98.0 to 102.0. % RSD should be not more than 2.0 at each % level. Results are depicted in Table 1.
| Levels |
Response 1 |
Response 2 |
Mean response |
Amount added |
Amount recovered |
%Recovery |
Mean % recovery |
%RSD |
| 80% |
865850 |
866480 |
866165 |
4.03 |
4.03 |
99.9 |
100.3 |
0.4 |
| 866070 |
864181 |
865125 |
4.00 |
4.02 |
100.6 |
| 864145 |
863678 |
863911 |
4.00 |
4.02 |
100.5 |
| 100% |
1078756 |
1073554 |
1076155 |
5.02 |
5.00 |
99.7 |
99.9 |
0.3 |
| 1072564 |
1074562 |
1073563 |
5.00 |
4.99 |
99.8 |
| 1075623 |
1074523 |
1075073 |
4.99 |
5.00 |
100.3 |
| 120% |
1300665 |
1304405 |
1302535 |
6.02 |
6.06 |
100.6 |
101.0 |
0.3 |
| 1307222 |
1302634 |
1304928 |
6.01 |
6.07 |
101.0 |
| 1310882 |
1307154 |
1309018 |
6.01 |
6.09 |
101.3 |
Table 1: Accuracy data of Anagrelide.
Precision
The percentage assay of six replicate injections of the drugs were performed and the % RSD of individual drugs were calculated and found to be 0.8 which are found to be within the limits. Results are depicted in Table 2.
| Sample determination |
Assay value %w/w |
| Preparation 1 |
99.6 |
| Preparation 2 |
101.6 |
| Preparation 3 |
99.4 |
| Preparation 4 |
99.8 |
| Preparation 5 |
100.1 |
| Mean |
100.0 |
| %RSD |
0.8 |
Table 2: Method precision data.
Linearity
The linearity of the method was tested for Anagrelide at 5 concentration levels and the correlation coefficient was calculated for both the drugs and found to be within limits i.e., correlation coefficient 0.99. The calibration graph was found to be linear in the range of 4-12 μg/ml of concentrations. Results are given in Table 3. And the graph was given in Figures 1-3.
Figure 1: Structure of Anagrelide HCl.
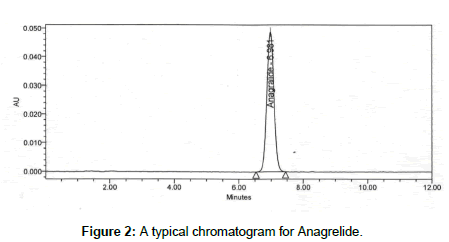
Figure 2: A typical chromatogram for Anagrelide.
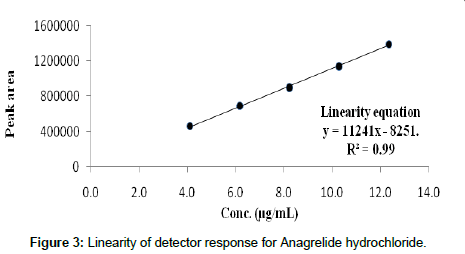
Figure 3: Linearity of detector response for Anagrelide hydrochloride.
| Level |
Concentration (µg/ml) |
RT |
Response |
| Level-1 |
4.1 |
6.98 |
461443 |
| Level-2 |
6.2 |
6.98 |
688968 |
| Level-3 |
8.2 |
6.98 |
900077 |
| Level-4 |
10.3 |
6.98 |
1141007 |
| Level-5 |
12.3 |
6.98 |
1391482 |
Table 3: Linearity of detector response for Anagrelide.
LOD and LOQ
Limit of detection is the minimum amount of analyte that can be detected but not necessarily quantified. Limit of quantitation can be defined as the lowest amount of analyte that can quantified. LOD and LOQ can be determined by the analysis of samples with known concentrations of analyte. The LOD was found to be 2.99 and LOQ was found to be 9.97 The values of LOD and LOQ were shown in the Table 4.
| Parameter |
Concentration (µg/mL) |
RT |
Response |
S/N Ratio |
| LOD |
0.043 |
6.97 |
3350 |
2.99 |
| LOQ |
0.13 |
6.99 |
10850 |
9.97 |
Table 4: LOD and LOQ Response for Anagrelide.
Specificity
There was no absolute interference from placebo preparations at retention time of Anagrelide Hcl. This shows that the method is specific.
System suitability
The system suitability parameters were evaluated. The %RSD for five replicate injections of Anagrelide Hcl was 0.2 respectively and found to be within the limits. The tailing factors for Anagrelide Hcl was found to be 1.09 respectively. The theoretical plates for Anagrelide Hcl was found to be 5144 and found to be within the limits. The system suitability parameters were evaluated and found to be within the limits. Results are depicted in Tables 5 and 6.
| Injection. No. |
Retention time |
Standard responses for Anagrelide peak |
Acceptance criteria |
| 1 |
6.98 |
897553 |
%RSD should be NMT 2.0 |
| 2 |
6.97 |
898663 |
| 3 |
6.98 |
898772 |
| 4 |
6.98 |
899138 |
| 5 |
6.98 |
901182 |
| 6 |
6.99 |
900670 |
| Mean |
899329.7 |
| Standard deviation |
1354.721 |
| %RSD |
0.2 |
System Precision and system suitability.
Table 5: System precision data.
| System suitability parameters |
Results |
Acceptance criteria |
| The % relative standard deviation for area of Anagrelide for five replicate injections |
0.18 |
NMT 2.0 |
| The theoretical plates of Anagrelide |
5144 |
NLT 2000 |
| The tailing factor for Anagrelide peak |
1.09 |
NMT 2.0 |
| % recovery of Check standard |
99.4 |
98.0-102.0 |
Table 6: System suitability data.
Forced degradation
In acid degradation 0.1 N HCl, heated on Water bath for 60 minutes @ 60°C % net degradation is 2.4 the peak purity is 1.0000. In base degradation 0.2 N NaOH heated on water bath for 60 minutes @ 60°C the % net degradation is 2.7 the peak purity is 1.0000. In water degradation water heated on water bath for 60 min @ 60°C % net degradation is 1.7 and peak purity is 1.0000. In thermal degradation 24 Hours @ 105°C there is no % net degradation and peak purity is 1.0000. In UV light degradation 4.00 Days at 200 watts/m2/hr there is no % net degradation. In visible light degradation 4.00 days at 1.2 million lux hours there is 0.0% net degradation. In humidity degradation, the % degradation is 0.1 and peak purity is 1.0000.
Conclusion
A new RP-HPLC method was developed for the estimation of Anagrelide Hydrochloride in bulk and pharmaceutical dosage form. the developed method can be used for routine quality control analysis and stability studies of Anagrelide Hydrochloride.
20408
References
- ICH Harmonised Tripartite Guideline (2003) Stability Testing of New Drug Substances and Products Q1A (R2).
- Anagrelide Official FDA Information: Side Effects and Uses. Available from: https://www.drugs.com/pro/anagrelide.html
- Pujeri SS, Khader A, Seetharamappa J (2012) Development and validation of a stability-indicating RP-HPLC method for the quantitative analysis of anagrelide hydrochloride. Scientia Pharmaceutica 80: 567-580.
- Kalyani N, Archana A, Sridhar S (2014) A validated UV Spectroscopic method for the determination Anagrelide in bulk & tablet dosage forms. Int J Pharm 4: 317-320.
- Rajendra Prasad Y, Neelima B, Nageswara Rao P, Hima Bindu V (2014) A novel LC-ESI-MS/MS assay method for the determination of Anagrelide in human plasma by using a solid phase extraction technique and its application to a pharmacokinetic study. Anal Methods 6: 4262-4270.
- Kalaichelvi R, Jayachandran E (2013) Development and validation of a reversed-phase HPLC method for the determination of Anagrelide in capsule. International Journal of Pharmaceutical Chemistry 3: 249-251.
- Kakadiya B, Gurmeet S (2013) Development and validation of a Stability-indicating RP-HPLC method for the determination of Anagrelide hydrochloride in pharmaceutical formulation. International Journal of Pharmaceutical & Biological Archives 4: 342-346.









