Research Article - (2024) Volume 18, Issue 6
Micro-acoustic Transmitter Implantation Impacts Juvenile Rainbow Trout Oncorhynchus mykiss Growth, Hematocrit and Splenosomatic Index
Alayna Wagner1,
Nathan Huysman1*,
Jeremy Kientz2,
Jill M. Voorhees1 and
Michael E. Barnes1
1McNenny State Fish Hatchery, South Dakota Game, Fish and Parks, Trout Loop Spearfish, South Dakota, United States
2South Dakota Game, Fish and Parks, Adventure Trail Rapid City, South Dakota, United States
*Correspondence:
Nathan Huysman, McNenny State Fish Hatchery, South Dakota Game, Fish and Parks, Trout Loop Spearfish, South Dakota,
United States,
Email:
Received: 20-Nov-2024, Manuscript No. IPFS-24-15359;
Editor assigned: 25-Nov-2024, Pre QC No. IPFS-24-15359 (PQ);
Reviewed: 09-Dec-2024, QC No. IPFS-24-15359;
Revised: 18-Dec-2024, Manuscript No. IPFS-24-15359 (R);
Published:
25-Dec-2024
Abstract
Micro-acoustic transmitters are becoming increasingly
popular in fisheries management. This study examined the
short-term effects of micro-transmitter surgical insertion on
juvenile rainbow trout Oncorhynchus mykiss (mean (SE)
initial weight=23.9 (1.8) g, length=124 (2) mm). One group
of trout were anesthetized and surgically implanted with
micro-acoustic transmitters (tag-to-body ratio=2.94 (0.07)
%). A second control group only underwent anesthesia. Ten
fish from each group were placed in one of five
experimental tanks for eight weeks, with weight, length,
hematocrit, hepatosomatic index, viscerosomatic index and
splenosomatic index data collected weekly. Survival in the
untagged control group was 100%, which was significantly
greater than 91.8% in the tagged group. Tag retention was
71%. Total lengths and weights were significantly less for the
first six weeks after surgery in tagged fish compared to the
control fish. Hematocrit was significantly lower and
splenosomatic index was significantly higher in the tagged
trout for the first three weeks. Hepatosomatic index and
viscerosomatic index were not significantly different
between the groups throughout the study. This study
provides additional documentation of the potential negative
effects of micro-acoustic tag implantation on juvenile
rainbow trout. A minimum three-week recovery period is
recommended for juvenile fish tagged at a 2.9% tag-to-body
ratio.
Keywords
Rainbow trout; Oncorhynchus mykiss; Microacoustic
transmitter; Surgery
Introduction
Acoustic transmitters have been used in fisheries
management to study behavior, survival and migration patterns
of numerous fish species [1-6]. For acoustic transmitter data to
be accurate and reliable, post-tagging behavior, physiology,
growth and immune function of acoustically tagged fish must be
similar to untagged fish [7-9]. However, this has not been
observed in several studies.
Cameron et al. reported significantly reduced survival for subyearling
Chinook salmon Oncorhynchus tshawytscha tagged with
micro-acoustic transmitters [10]. Micro-acoustic tagged Chinook
salmon exposed to rapid decompression experienced
significantly reduced survival compared to untagged
counterparts [11]. Furthermore, migrating Chinook salmon with
micro-acoustic tags have reduced survival, longer downstream
migration times and experience heightened inflammation inside
the body cavity compared to untagged fish from the same group
[9]. Lastly, a significant and large decrease in hematocrit,
indicating an anemic response, was observed in juvenile rainbow
trout Oncorhynchus mykiss for at least 30 days after
implantation of dummy micro-acoustic tags [12].
The duration of anemia in micro-acoustic-tagged trout is
unknown. Millsap et al. [12] experiment lasted only 30 days and
hematocrit in the tagged trout did not return to basal levels by
the end of the experiment. However, the mean tag-to-body ratio
used by Millsap et al. [12] was 4.8%. Although this is more than
the 2% ratio recommended by Winter over 25 years ago, it is
well within the higher tag-to-body ratios currently being used
with small salmonids [13-21]. It is unknown if these negative
effects of tagging on hematocrit occur in trout at lower tag-tobody
ratios.
This study had two objectives involving rainbow trout closer
to a 2% tag-to-body ratio. The first objective was to determine
the time required for complete recovery of anemia in microacoustically
tagged rainbow trout. The second objective was to
assess the potential impacts of micro-acoustic tagging on
relatively larger juvenile rainbow trout to growth and
morphological indices.
Materials and Methods
All experimentation occurred at McNenny State Fish Hatchery,
rural Spearfish, South Dakota, USA, using degassed and aerated
well water (11°C; total hardness 360 mg/L CaCO3; alkalinity as
CaCO3 210 mg/L; pH 7.6, total dissolved solids 390 mg/L). This
study used 98 Arlee strain rainbow trout. These fish arrived at
the hatchery as eyed eggs on 23 November 2022 and had been
on feed for approximately 150 days prior to use in the experiment. Experimentation occurred in five 190-L semi square
tanks. The top of each tank was partially covered with
corrugated black plastic and the remaining opening covered with
netting to prevent fish escapement [22].
All the fish were anesthetized with 60 mg/L tricaine methane
sulfonate (MS-222, Syndel; Ferndale, Washington, USA). After
approximately five minutes in anesthetic solution, each fish was
weighed to the nearest 0.1 g and measured (total length) to the
nearest mm. One-half of the fish (n=49) were then surgically
implanted with a dummy acoustic transmitter (Innovasea V5,
Nova Scotia, Canada; weight=0.7 g, length=12.7 mm,
diameter=4.3 × 5.7 mm). To minimize potential surgeon bias, a
single, experienced surgeon conducted all the surgeries [23].
Fish undergoing surgery were placed ventral side up in a
grooved sponge and an incision was made just large enough for
transmitter insertion. The location of the incision was beside the
mid-central line and anterior to the pelvic girdle (Figure 1).
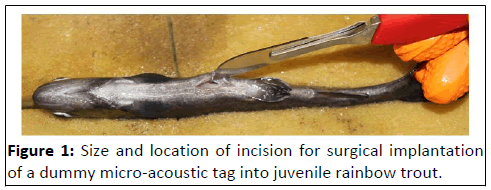
Figure 1: Size and location of incision for surgical implantation
of a dummy micro-acoustic tag into juvenile rainbow trout.
After dummy transmitter insertion (Figures 2 and 3), the incision
was closed with one suture (PDO II Violet monofilament absorbable
polydioxanone suture, Oasis; Mettawa, Illinois, USA). After surgery,
the tagged fish were placed in a recovery tank of fresh water.
Anesthetized-only control fish were also placed in a recovery tank
after five minutes of anesthesia, which was the typical duration of
anesthesia for the tagged fish. After the fish recovered from
anesthesia and were freely swimming, they were placed into one of
the five tanks. Because of a limited number of dummy transmitters,
four of the five tanks received 20 fish (10 tagged, 10 untagged), with
one tank receiving 18 fish (9 tagged, 9 untagged).

Figure 2: Insertion of dummy micro-acoustic tag into the
coelomic cavity through incision of a juvenile rainbow trout.
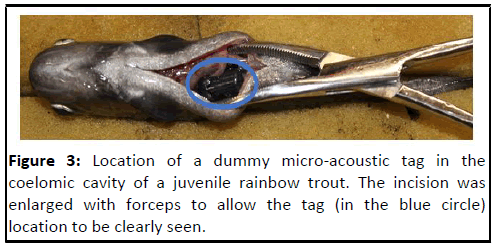
Figure 3: Location of a dummy micro-acoustic tag in the
coelomic cavity of a juvenile rainbow trout. The incision was
enlarged with forceps to allow the tag (in the blue circle)
location to be clearly seen.
Every seven days and at the end of the experiment (day 56),
all the fish were anesthetized, measured to the nearest mm
(total length) and weighed to the nearest 0.1 g. One randomly
selected experimental fish and one randomly selected control
fish from each tank were euthanized with a lethal dose of 200
mg/L MS-222. Following euthanasia, the caudal fin was severed.
Blood was collected in a heparinized microhematocrit capillary
tube (Fisher Scientific; Pittsburg, Pennsylvania) and sealed with
sealant (Critoseal, Oxford Labware, Sherwood Medical Products,
St. Louis, Missouri, USA). The capillary tube was then centrifuged
at 11,500 rpm for 10 minutes to separate the volume of red
blood cells from blood plasma. A digital caliper was used to
measure the red blood cell and the total blood volume in the
capillary tube to the nearest 0.01 mm [24]. These values were
used to directly calculate hematocrit [25]. The liver, viscera and
spleen were removed from the fish and weighed to the nearest
0.0001 g. Hematocrit, Hepatosomatic Index (HSI), Viscerosomatic
Index (VSI), Splenosomatic Index (SSI) and Specific Growth Rate
(SGR) values were obtained using the following formulas:
Hematocrit (%)=(red⁄whole blood) × 100
Hepatosomatic Index (HSI)=(liver weight (mg)⁄total weight
(mg)) × 100
Viscerosomatic Index (VSI)=(viscera weight (mg)⁄total weight
(mg)) × 100
Splenosomatic Index (SSI)=(spleen weight (mg)⁄total weight
(mg)) × 100
Specific Growth Rate (SGR)=[(ln(end weight)-ln(start
weight))⁄number of days)] × 100
Each tank of fish received 1.5 mm extruded feed (Protec,
Skretting; Tooele, Utah, USA) using automatic feeders (Pentair
AES AVF6; Cary, North Carolina, USA). Fish were fed to apparent
satiation. Feeding did not occur on the day of data collection
each week. The experiment lasted for a total of eight weeks
post-surgery.
Data were analyzed using the SPSS (24.0) statistical analysis
program (IBM, Armonk, New York, USA) with significance
predetermined at P<0.05. A repeated measures analysis of
variance ANOVA was used to determine if differences occurred
between the untagged (control) and tagged groups over the
course of the study for weight, length, hematocrit,
hepatosomatic index, viscerosomatic index and splenosomatic
index. The tanks were the experimental unit and the fish were a
fixed effect. Mauchly’s sphericity test was used to test for equal
variances. If variances were unequal, a Huynh-Feldt correction
was used. If the repeated measures ANOVA indicated overall
significant differences between the tagged and untagged fish, a
post-hoc test t-test was conducted at each weekly time point.
Specific growth rate values were negative for the first week of
the experiment, negating the use of repeated measures ANOVA.
Instead, a t-test was used to analyze specific growth rate data for
each week. Chi-square analysis was used to determine if there
was a significant difference in percent survival between the
tagged and untagged groups.
Results
At 91.8%, survival was significantly lower (P=0.041) in
the tagged fish compared to the 100% survival in the
untagged control fish. Tag retention was 71%, with three tags
lost in the second week, one tag lost in the fourth week, four
tags lost in the fifth week, three tags lost in the sixth week
and three tags lost in the seventh week.
The initial mean (SE) weight and total length of the rainbow
trout used in this study were 23.9 (1.8) g and 124 (2)
mm, respectively. This fish size in relation to transmitter size
resulted in an initial mean (SE) tag-to-body ratio of 2.94 (0.07)
% in the surgically implanted trout. There was a significant
difference in length (F4.54, 36.28=4.72, P=0.003) and weight
(F4.87, 38.92=2.56, P=0.044) between the tagged and
untagged groups of trout over the course of the trial
(Figures 4 and 5). The control, anesthetized-only
fish weighed significantly more than fish implanted with
dummy acoustic transmitters at the end of the first, second,
third, fifth and sixth weeks. Similarly, control fish were
significantly longer than tagged fish at the end of the first, third,
fifth and sixth weeks.
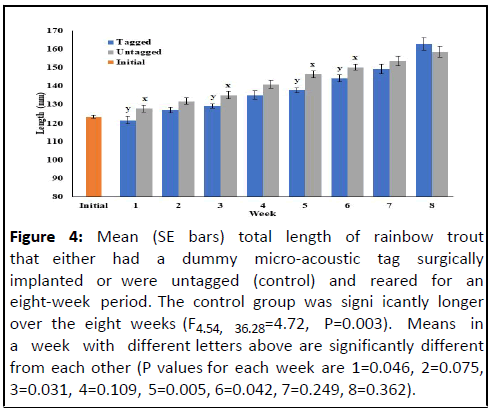
Figure 4: Mean (SE bars) total length of rainbow trout
that either had a dummy micro-acoustic tag surgically
implanted or were untagged (control) and reared for an
eight-week period. The control group was signi icantly longer
over the eight weeks (F4.54, 36.28=4.72, P=0.003). Means in
a week with different letters above are significantly different
from each other (P values for each week are 1=0.046, 2=0.075,
3=0.031, 4=0.109, 5=0.005, 6=0.042, 7=0.249, 8=0.362).
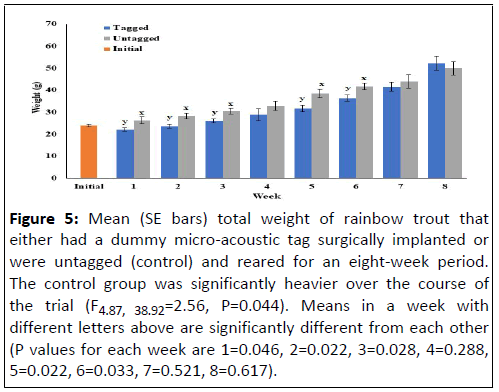
Figure 5: Mean (SE bars) total weight of rainbow trout that
either had a dummy micro-acoustic tag surgically implanted or
were untagged (control) and reared for an eight-week period.
The control group was significantly heavier over the course of
the trial (F4.87, 38.92=2.56, P=0.044). Means in a week with
different letters above are significantly different from each other
(P values for each week are 1=0.046, 2=0.022, 3=0.028, 4=0.288,
5=0.022, 6=0.033, 7=0.521, 8=0.617).
Specific growth rate was highly variable, with large standard
errors at each sampling period (Figure 6). It was significantly
different between the groups at the end of the first week, with
negative values for the surgically implanted fish compared to
positive values for the untagged control fish. While specific
growth rate was positive for both groups of fish through the
remainder of the experiment, there were no significant
differences observed between the tagged and untagged fish.
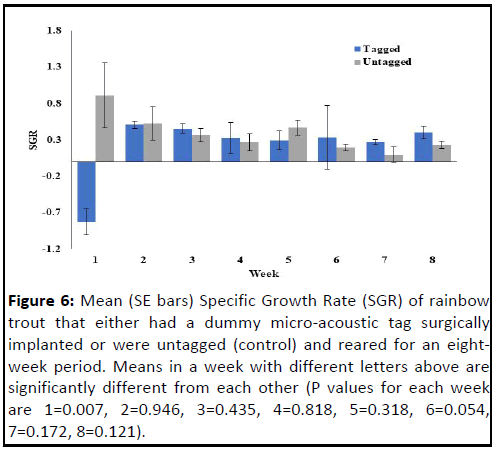
Figure 6: Mean (SE bars) Specific Growth Rate (SGR) of rainbow
trout that either had a dummy micro-acoustic tag surgically
implanted or were untagged (control) and reared for an eightweek
period. Means in a week with different letters above are
significantly different from each other (P values for each week
are 1=0.007, 2=0.946, 3=0.435, 4=0.818, 5=0.318, 6=0.054,
7=0.172, 8=0.121).
Hematocrit was significantly lower in the tagged fish compared
to the untagged fish over the course of the trial (F8.0, 40=2.82,
P=0.01) (Figure 7). Significant differences occurred at the end of
the first, second and third weeks of the experiment. Hematocrit
was not significantly different between the two groups during the
remainder of the experiment with levels stabilizing near basal for
both treatments.
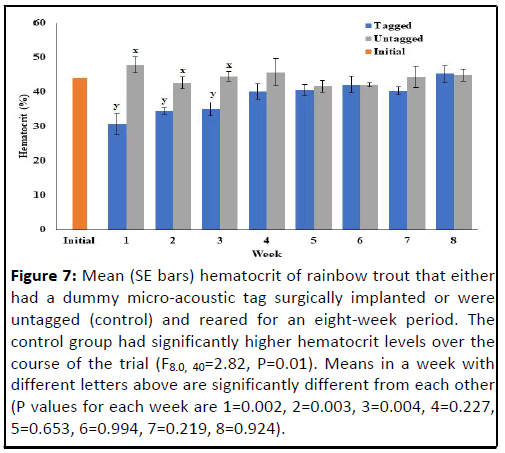
Figure 7: Mean (SE bars) hematocrit of rainbow trout that either
had a dummy micro-acoustic tag surgically implanted or were
untagged (control) and reared for an eight-week period. The
control group had significantly higher hematocrit levels over the
course of the trial (F8.0, 40=2.82, P=0.01). Means in a week with
different letters above are significantly different from each other
(P values for each week are 1=0.002, 2=0.003, 3=0.004, 4=0.227,
5=0.653, 6=0.994, 7=0.219, 8=0.924).
Neither hepatosomatic index (F8.0, 64=0.91, P=0.51) nor
viscerosomatic index (F8.0, 64.0=0.59, P=0.78) were significantly
different between the tagged and untagged fish throughout the
experiment (Figures 8 and 9). However, splenosomatic index
was significantly different between the groups (F5.48, 43.85=4.59,
P=0.001) (Figure 10). At the end of the first, second and third
weeks, splenosomatic index was significantly higher in the fish
with surgically implanted transmitters compared to untagged
control fish. In the fourth, fifth, sixth and seven weeks after the
start of the experiment, splenosomatic index was not
significantly different between the tagged and untagged fish.
However, at the end of the experiment (eighth week), the
splenosomatic index was again significantly higher in the tagged
group compared to the control group. In general, over the
duration of the experiment, splenosomatic index in the tagged
fish group was highly variable and never appeared to stabilize.
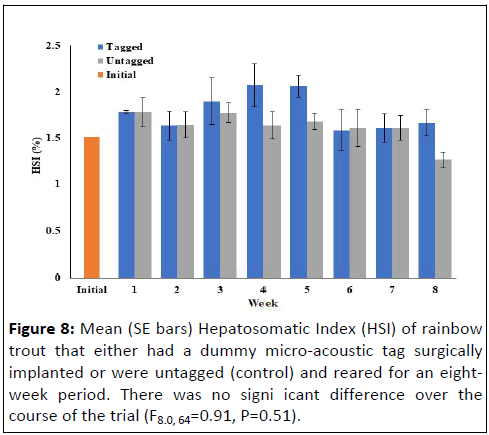
Figure 8: Mean (SE bars) Hepatosomatic Index (HSI) of rainbow
trout that either had a dummy micro-acoustic tag surgically
implanted or were untagged (control) and reared for an eightweek
period. There was no signi icant difference over the
course of the trial (F8.0, 64=0.91, P=0.51).
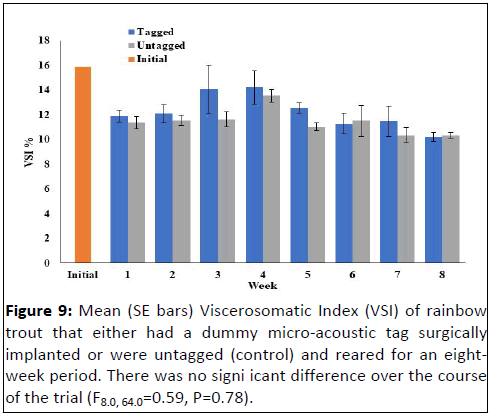
Figure 9: Mean (SE bars) Viscerosomatic Index (VSI) of rainbow
trout that either had a dummy micro-acoustic tag surgically
implanted or were untagged (control) and reared for an eightweek
period. There was no signi icant difference over the course
of the trial (F8.0, 64.0=0.59, P=0.78).
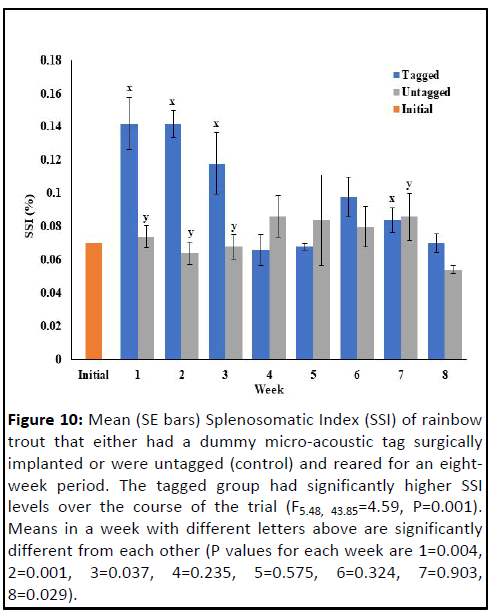
Figure 10: Mean (SE bars) Splenosomatic Index (SSI) of rainbow
trout that either had a dummy micro-acoustic tag surgically
implanted or were untagged (control) and reared for an eightweek
period. The tagged group had significantly higher SSI
levels over the course of the trial (F5.48, 43.85=4.59, P=0.001).
Means in a week with different letters above are significantly
different from each other (P values for each week are 1=0.004,
2=0.001, 3=0.037, 4=0.235, 5=0.575, 6=0.324, 7=0.903,
8=0.029).
Discussion
The results of this study showing reduced hematocrit,
increased splenosomatic index and reduced growth in juvenile
rainbow trout for three weeks after tag implantation indicates
that micro-acoustic tagging can have substantial negative shortterm
effects. These results support those of Millsap et al., who
observed a similar large decrease in hematocrit in a smaller size
class of rainbow trout [12]. Heightened inflammatory responses
and poor body-conditions have also been reported in juvenile
Chinook salmon tagged with micro-acoustic transmitters [9].
Hematocrit is the ratio of red blood cells to whole blood
volume. A reduced hematocrit is indicative of anemia, which
results in a reduced capacity to transport oxygen and
subsequent negative impacts on energy utilization [26].
Transmitter implantation is highly invasive [27]. The 25%
reduction in hematocrit observed in tagged fish for 21 days in
this study indicates micro-acoustic tag implantation was an
acute stress event for the fish [28,29]. Thus, it is problematic to
assume that recently tagged fish are similar to untagged
conspecifics. A 22% reduction in hematocrit has been shown to
significantly reduce critical swimming velocities and maximal
oxygen uptakes in fish [30]. Millsap et al. reported a 50%
reduction in hematocrit a week after implanting micro-acoustic
tags in rainbow trout, with hematocrit levels never reaching
control fish levels during the four-week experiment. Because predation tags have a relatively short battery life, implantation
typically occurs shortly before release of the fish, at a time when
the fish are most likely still anemic [6,21,31].
It is unknown if the large decrease in hematocrit observed in
this study is from blood loss during the surgical process or is a
stress response from the tag itself. However, surgery of
pikeperch Sander lucioperca insertion of radio transmitters with
a tag burden of less than 1.2% body weight did not result in a
reduction of hematocrit compared to control fish [32]. Typically,
hematocrit reductions in fish are due to parasites, infections,
toxins or heavy metals in the water [33-39]. It is possible that the
rainbow trout used in this study acted as if the tag was a foreign
parasite or infection and increased white blood cell production
to counteract this perceived threat.
In simplest terms, specific growth rate is the percentage increase
in weight per day based on the exponential growth typically
observed in smaller fish, like those used in this study [40].
Negative specific growth rates indicate weight loss [41].
Weight loss during the first week after tag implantation surgery in
fish has been previously reported [42]. It is possible the negative
specific growth rate observed in the tagged trout during the first
week of this study could be because the fish either ceased or
decreased food consumption because of the post-surgery
inflammatory response or a combination of these factors
[9,43]. However, Robertson et al. reported decreased growth
but no difference in food consumption after transmitter
implantation surgery in Atlantic salmon Salmo salar parr,
indicating that it is more likely weight loss in the current study
was a direct result of the post-surgery healing process [44].
Other studies using acoustic tags have observed adverse
impacts on the growth of fish. For example, acoustically tagged
rainbow trout grew slower for at least 38 days compared to
untagged rainbow trout [45]. A similar decrease in growth was
observed in acoustically tagged juvenile Atlantic salmon [44,46]
and brook trout Salvelinus fontinalis [20]. Growth was not
reduced in tagged sockeye salmon Oncorhynchus nerka, but
swimming performance was reduced compared to sham and
control treatments [47]. Similar results have been documented
in coho salmon Oncorhynchus kisutch [16], Chinook salmon [17]
and Atlantic salmon [44]. In a four-week study with juvenile
rainbow trout, growth rates were lower throughout the study in
the tagged fish compared to untagged controls [12].
The spleen in fish is directly involved in immune function and
is the site of antibody production [48]. As such, the
splenosomatic index is an indicator of both the immune status of
the fish and its hematopoietic capacity [49-51]. Splenosomatic
index values of the control (untagged) fish in the current study
were within the range reported by numerous other studies
involving rainbow trout [52-56]. However, the splenosomatic
index of the tagged fish at both the start and end of the current
study are much higher than those previously reported, indicating
physiological stress in the fish receiving predation tags. It is
unknown if the increase in relative spleen size in the tagged fish
was a response to anemia or an indicator of challenges to the
immune system.
Nearly 30 years ago, Winter [13] recommended a maximum
2% tag burden in relation to the total weight of the fish.
Subsequent studies have successfully pushed well beyond that
limit and it is no longer well-supported [9,15,19-21,57,58]. Thus,
it is not surprising that the 2.9% tag-to-body ratio used in this
study produced similar results to the 4.8% tag-to-body ratio used
by Millsap et al [12]. It was also within the range of tag sizes used
by Wargo Rub et al. [9] who observed similar results. It should be
noted however, that there have been conflicting results
associated with similar tag-to-body ratios. Lennox et al. [21]
found no significant difference in migration for Atlantic salmon
smolts at 5.8% tag-to-body ratio, but a highly extensive study by
Wargo Rub et al. [19] found lower survival and increased
migration times for acoustically tagged Chinook salmon with a
mean tag-to-body ratio of 2.3%. Similarly, Smircich and Kelly [20]
reported no difference in swimming performance in brook trout
with tag burdens up to 7%, while Perry et al. [59] reported
reduced swimming performance of juvenile Chinook salmon at a
tag burden range from 3.4%-4.0%.
The lack of significant differences in hepatosomatic index
between the tagged and untagged fish in this study indicates
that tag implantation and surgery did not impact subsequent
energy partitioning. Hepatosomatic index indirectly measures
glycogen and carbohydrate levels and indicates the nutritional
status of the fish [50,60-62]. The similar viscerosomatic index
levels in the tagged and untagged fish indicate that tag
implantation and surgery did not affect lipid metabolism [63-66].
It is possible, but unlikely, that the co-rearing of tagged and
untagged fish may have influenced the results of this study.
Rearing both groups of fish in the same tank was done to provide
replication and because it is how tagged fish would typically be
handled in a hatchery setting. Production hatcheries usually do
not have the space to maintain tagged fish in a separate rearing
unit or place individually tagged fish in their own discrete rearing
units after surgery. Thoreau and Baras [67] rejected the idea that
untagged fish somehow impaired the recovery of tagged tilapia
Oreochromis aureus. In contrast to domesticated rainbow trout,
Tilapia are much more territorial [68,69]. In addition, the rearing
densities used in the current study were low. The maximum
density index was only 0.32, which is well below the typical
recommendations of 0.5-1.0 for rainbow trout [70].
Lietdtke et al. [71] recommending holding fish for up to 36
hours after surgical tag implantation before transport or
stocking. Thoreau and Baras [67] recommended doubling the
recovery period to 72 hours. However, both time frames are
likely much too soon to release micro-acoustic tagged fish.
Mortality and tag expulsion could occur well after this period,
with fish stress remaining high for up to 168 hours post-stocking
[72]. In addition, the stressful effects of loading and stocking
[73,74] in combination with post-tagging anemia would be very
problematic. Thus, the behavior and survival [74] of any fish
released less than 30 days after tagging would most likely not be
representative of untagged individuals, rendering any tagging
data collected prior to 30 days inaccurate and unreliable [12].
The 75% tag retention observed in this study was
nearly identical to the 76% retention reported by Millsap et al.
[12] in a similar study using smaller rainbow trout. Tag
retention in this study was also similar to the 73% reported
by Urbaniak et al. [45] and the 78% reported by Kientz et al
[76]. Tag expulsion occurred through the incision site, where it
was likely enabled by the loss of skin integrity and inflammation
[77]. Just as Millsap et al. [12] reported, the fish that expelled
their tags remained alive for the duration of the experiment.
In the current study only tagged fish died, with the mortality
primarily occurring within the first two weeks after surgery.
The 91.8% survival of the tagged fish and 100% survival of the
control fish was also nearly identical to the 92% and 100%
survival rates reported with smaller rainbow trout by Millsap et
al [12].
The constant 11°C water temperature used in this study
produced a very favorable tagging environment for the
rainbow trout. Warmer temperatures, particularly above 17°C,
have led to decreased survival, poorer surgical wound healing
and poorer tag retention in tagged trout [9,78,79]. Higher
temperatures increase the inflammatory response and may
impact the intensity and longevity of the anemia observed in this
study [30,80,81].
The Innovasea V5 dummy acoustic tag used in this study can
be customized to collect different types of data. They are
increasingly being used as acid-sensitive predation sensors to
evaluate the survival of fish for a short time period after stocking
[6,82-84]. The results of this study, along with those of Wargo
Rub et al. and Millsap et al., [12] strongly suggest that the
information [9] obtained from these transmitters should be used
with caution. For example, Gravenhof et al. [6] estimated
predation rates for juvenile Chinook salmon stocked at either 5-6
days or 19-20 days after surgical implantation of predation tags
physically identical to those used in this and the Millsap et al. [12]
study. The short-term anemic response, which appears to last
longer in smaller fish, increased splenosomatic index and weight
loss or reduced growth would likely make the tagged fish more
vulnerable to predation, thereby negating the assumption that
they are representative of untagged fish. It cannot be assumed
that implanting micro-acoustic transmitters has a negligible effect
on the tagged fish.
Conclusion
This study documented the negative effects of decreased
hematocrit, reduced growth and potential immunological issues
associated with predation tag implantation on juvenile rainbow
trout. These results appear to invalidate the assumption that
untagged and tagged fish behave and survive similarly after
tagging. A minimum three-week recovery period is needed after
surgery for the recovery of fish surgically implanted with acoustic
tags. For wild fish tagged and immediately released, any data
collected for the first three weeks should either be disregarded
or used with extreme caution. More research in a controlled
environment is needed to determine the post-implantation
recovery times required for the additional species and sizes of
fish receiving acoustic tags.
Acknowledgments
Thanks to Alexis Gerber, Breah Rosners and Marissa Tuttle for
their assistance with this experiment.
References
- Lucas MC, Baras E. Migration of freshwater fishes. Blackwell Science, Oxford, 2001.
- Brown RS, Eppard MB, Murchie KJ, Nielsen JL, Cooke SJ (2011) An introduction to the practical and ethical perspectives on the need to advance and standardize the intracoelomic surgical implantation of electronic tags in fish. Rev Fish Biol Fish 21: 1-9.
[Crossref] [Google Scholar]
- Cooke SJ, Woodley CM, Brad Eppard M, Brown RS, Nielsen JL (2011) Advancing the surgical implantation of electronic tags in fish: A gap analysis and research agenda based on a review of trends in intracoelomic tagging effects studies. Rev Fish Biol Fish 21: 127-151.
[Crossref] [Google Scholar]
- Leber KM, Blankenship HL (2011) How advances in tagging technology improved progress in a new science: Marine stock enhancement. Ame Fish Soc Sym 76: 1-12.
[Google Scholar]
- Thorstad EB, Rikardsen AH, Alp A, Okland F (2013) The use of electronic tags in fish research-an overview of fish telemetry methods. Turk J Fish Aquat Sci 13: 881-896.
[Google Scholar]
- Gravenhof DA, Wuellner MR, Renner EA, Fincel MJ (2024) Estimating predation rates of stocked juvenile Chinook salmon using novel acoustic predation transmitters. N Am J Fish Manag 44: 438-448.
[Crossref] [Google Scholar]
- Anglea SM, Geist DR, Brown RS, Deters KA, McDonald RD (2004) Effects of acoustic transmitters on swimming performance and predator avoidance of juvenile Chinook salmon. N Am J Fish Manag 24: 162-170.
[Crossref] [Google Scholar]
- Panther JL, Brown RS, Gaulke GL, Deters KA, Woodley CM, et al. (2011) Influence of incision location on transmitter loss, healing, survival, growth and suture retention of juvenile Chinook salmon. Trans Am Fish Soc 140: 1492-1503.
[Crossref] [Google Scholar]
- Wargo Rub AM, Sandford BP, Butzerin JM, Cameron AS (2020) Pushing the envelope: Micro-transmitter effects on small juvenile Chinook salmon (Oncorhynchus tshawytscha). PLoS One 15: e0230100.
[Crossref] [Google Scholar] [PubMed]
- Cameron AS, Rub AM, Sandford BP (2023) Evaluation of healing progression at surgical incision sites and the use of antiseptics for enhancing post-operative survival in subyearling Chinook salmon (Oncorhynchus tshawytscha). PloS One 18: e0288056.
[Crossref] [Google Scholar] [PubMed]
- Boyd JW, Deters KA, Brown RS, Eppard MB (2011) Efficacy of single-suture incision closures in tagged juvenile Chinook salmon exposed to simulated turbine passage. Trans Am Fish Soc 140: 1186-1192.
[Crossref] [Google Scholar]
- Millsap EK, Huysman N, Gravenhof DA, Fincel MJ, Barnes ME (2023) Effects of predation tags on growth and stress response in juvenile rainbow trout Oncorhynchus mykiss. Hydrobiology 2: 467-474.
[Crossref] [Google Scholar]
- Winter JD (1996) Advances in underwater biotelemetry. 2nd edition, American Fisheries Society, Bethesda, pp. 555-590.
- Brown RS, Cooke SJ, Anderson WG, McKinley RS (1999) Evidence to challenge the 2% rule for biotelemetry. N Am J Fish Manag 19: 867-871.
[Crossref] [Google Scholar]
- Jepsen N, Schreck CB, Clements S, Thorstad EB (2005) A brief discussion on the 2% tag/bodymass rule of thumb. Food and Agriculture Organization of the United Nations, Italy, pp. 255-259.
- Chittenden CM, Butterworth KG, Cubitt KF, Jacobs MC, Ladouceur A, et al. (2009) Maximum tag to body size ratios for an endangered coho salmon (O. kisutch) stock based on physiology and performance. Environ Biol Fish 84: 129-140.
[Crossref] [Google Scholar]
- Brown RS, Harnish RA, Carter KM, Boyd JW, Deters KA, et al. (2010) An evaluation of the maximum tag burden for implantation of acoustic transmitters in juvenile Chinook salmon. N Am J Fish Manag 30: 499-505.
[Crossref] [Google Scholar]
- Rechisky EL, Welch DW (2010) Surgical implantation of acoustic tags: Influence of tag loss and tag-induced mortality on free ranging and hatchery-held spring Chinook (O. tschawytscha) smolts. PNAMP Special Publication, Washington, pp. 69-94.
- Wargo Rub AM, Gillbreath LG, McComas RL, Sandford BP, Teel DJ, et al. (2012) Survival of adult spring/summer Chinook salmon from the mouth of the Columbia River to Bonneville Dam, 2011. National Oceanic and Atmospheric Administration, Washington, USA.
- Smircich MG, Kelly JT (2014) Extending the 2% rule: The effects of heavy internal tags on stress physiology, swimming performance and growth in brook trout. Anim Biotelem 2: 1-7.
[Crossref] [Google Scholar]
- Lennox RJ, Stoger E, Dahlmo LS, Helle T, Wiers T, et al. (2022) Effects of tag type and surgery on migration of Atlantic salmon (Salmo salar) smolts. J Fish Biol 101: 515-521.
[Crossref] [Google Scholar] [PubMed]
- Barnes ME, Durben DJ (2003) Use of partial tank covers during hatchery rearing of feral rainbow trout. N Am J Aquac 65: 344-348.
- Heim KC, Withers J, Castro‐Santos T (2024) Tagger effects in aquatic telemetry: Short‐term and delayed effects of surgery in Atlantic Salmon smolts. N Am J Fish Manag 44: 262-275.
[Crossref] [Google Scholar]
- Kelly ME, Luetkemeier MJ, Pantalos GM (1994) A justification for high resolution hematocrit measurement. Med Sci Sports Exerc 26: 547-550.
[Google Scholar] [PubMed]
- Billett HH (1990) Hemoglobin and hematocrit. Clinical methods: The history, physical and laboratory examinations. 3rd edition, Boston.
[Google Scholar] [PubMed]
- Gallaugher P, Thorarensen H, Farrell AP (1995) Hematocrit in oxygen transport and swimming in rainbow trout (Oncorhynchus mykiss). Respir Physiol 102: 279-292.
[Crossref] [Google Scholar] [PubMed]
- Wilson AD, Hayden TA, Vandergoot CS, Kraus RT, Dettmers JM, et al. (2017) Do intracoelomic telemetry transmitters alter the post‐release behaviour of migratory fish? Ecol Freshwater Fish 26: 292-300.
[Crossref] [Google Scholar]
- Witeska M (2005) Stress in fish-hematological and immunological effects of heavy metals. Elect J Ichth 1: 35-41.
[Google Scholar]
- Burgos-Aceves MA, Lionetti L, Faggio C (2019) Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci Total Environ 670: 1170-1183.
[Crossref] [Google Scholar] [PubMed]
- Gallaugher P, Farrell AP (1998) Hematocrit and blood oxygen-carrying capacity. Fish Physiol 17: 185-227.
[Crossref] [Google Scholar]
- Lennox RJ, Nilsen CI, Nash A, Hanssen EM, Johannesen HL, et al. (2021) Laboratory and field experimental validation of two different predation sensors for instrumenting acoustic transmitters in fisheries research. Fisheries 46: 565-573.
[Crossref] [Google Scholar]
- Rozynski M, Kapusta A, Demska-Zakes K, Hopko M, Sikora A, et al. (2017) The effects of surgically implanted dummy tags on the survival, growth performance and physiology of pikeperch (Sander lucioperca). Fish Physiol Biochem 43: 999-1010.
[Crossref] [Google Scholar] [PubMed]
- Herman RL (1970) Effects of gossypol on rainbow trout Salmo guirdneri Richardson. J Fish Biol 2: 293-303.
[Crossref] [Google Scholar]
- Woo PT (1979) Trypanoplasma salmositica: Experimental infections in rainbow trout, Salmo gairdneri. Exp Parasitol 47: 36-48.
[Crossref] [Google Scholar] [PubMed]
- Haux C, Larsson A (1984) Long-term sublethal physiological effects on rainbow trout, Salmo gairdneri, during exposure to cadmium and after subsequent recovery. Aquat Toxicol 5: 129-142.
[Crossref] [Google Scholar]
- Tewari H, Gill TS, Pant J (1987) Impact of chronic lead poisoning on the hematological and biochemical profiles of a fish, Barbus conchonius (Ham). Bull Environ Contam Toxicol 38: 748-752.
[Crossref] [Google Scholar] [PubMed]
- Lemly AD (2002) Symptoms and implications of selenium toxicity in fish: The Belews lake case example. Aquat Toxicol 57: 39-49.
[Crossref] [Google Scholar] [PubMed]
- Martins ML, Tavares-Dias M, Fujimoto RY, Onaka EM, Nomura DT (2004) Haematological alterations of Leporinus macrocephalus (Osteichtyes: Anostomidae) naturally infected by Goezia leporini (Nematoda: Anisakidae) in fish pond. Arq Bras Med Vet Zootec 56: 640-646.
[Crossref] [Google Scholar]
- Ziskowski J, Mercaldo-Allen R, Pereira JJ, Kuropat C, Goldberg R (2008) The effects of fin rot disease and sampling method on blood chemistry and hematocrit measurements of winter flounder, Pseudopleuronectes americanus from New Haven Harbor (1987-1990). Mar Pollut Bull 56: 740-750.
[Crossref] [Google Scholar] [PubMed]
- Lugert V, Thaller G, Tetens J, Schulz C, Krieter J (2016) A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev Aquac 8: 30-42.
[Crossref] [Google Scholar]
- Deverill JI, Adams CE, Bean CW (1999) Prior residence, aggression and territory acquisition in hatchery‐reared and wild brown trout. J Fish Biol 55: 868-875.
[Crossref] [Google Scholar]
- Peressin A, Lopes JD, Bedore AG, Alves CB, Prado IG, et al. (2021) Radiotagging a long-distance migratory Characiform fish: Reproduction after surgery, tag losses and effects in weight. Neotrop Ichthyol 19: e200097.
[Google Scholar]
- DiMaria-Ghalili RA, Sullivan-Marx EM, Compher C (2014) Inflammation, functional status and weight loss during recovery from cardiac surgery in older adults: A pilot study. Biol Res Nurs 16: 344-352.
[Crossref] [Google Scholar] [PubMed]
- Robertson MJ, Scruton DA, Brown JA (2003) Effects of surgically implanted transmitters on swimming performance, food consumption and growth of wild Atlantic salmon parr. J Fish Biol 62: 673-678.
[Crossref] [Google Scholar]
- Urbaniak TJ, Barnes ME, Davis JL (2016) Acoustic transmitters impact rainbow trout growth in a competitive environment. Open Fish Sci J 9: 37-44.
[Google Scholar]
- Lacroix GL, Knox D, McCurdy P (2004) Effects of implanted dummy acoustic transmitters on juvenile Atlantic salmon. Trans Am Fish Soc 133: 211-220.
[Crossref] [Google Scholar]
- Brown RS, Geist DR, Deters KA, Grassell A (2006) Effects of surgically implanted acoustic transmitters >2% of body mass on the swimming performance, survival and growth of juvenile sockeye and Chinook salmon. J Fish Biol 69: 1626-1638.
[Crossref] [Google Scholar]
- Shibasaki Y, Afanasyev S, Fernandez-Montero A, Ding Y, Watanabe S, et al. Cold-blooded vertebrates evolved organized germinal center-like structures. Sci Immunol 8: eadf1627.
[Crossref] [Google Scholar] [PubMed]
- Smith LS (1991) Introduction to fish physiology. Argent Laboratories, Redmond, Washington.
- Barton BA, Morgan JD, Vijayan MM (2002) Physiological and condition-related indicators of environmental stress in fish. American Fisheries Society, Maryland, pp. 111-148.
[Google Scholar]
- Sharma J, Dar SA, Langer S, Sayani AN (2017) Seasonal variations in the Spleen Somatic Index (SSI) of Garra gotyla gotyla. J Entomol Zool Stud 5:173-175.
[Google Scholar]
- Parker TM, Barnes ME (2015) Effects of different water velocities on the hatchery rearing performance and recovery from transportation of rainbow trout fed two different rations. Trans Am Fish Soc 144: 882-890.
[Crossref] [Google Scholar]
- Kientz JL, Barnes ME (2016) Structural complexity improves the rearing performance of rainbow trout in circular tanks. N Am J Aquac 78: 203-207.
[Crossref] [Google Scholar]
- Bruce TJ, Sindelar SC, Voorhees JM, Brown ML, Barnes ME (2017) Performance and immunological responses of rainbow trout (Oncorhynchus mykiss) fed bioprocessed plant‐based proteins. Aquac Nutr 23: 1160-1168.
[Crossref] [Google Scholar]
- Voorhees JM, Barnes ME, Chipps SR, Brown ML (2018) Dietary bioprocessed soybean meal does not affect the growth of exercised juvenile rainbow trout (Oncorhynchus mykiss). J Anim Res Nutr 3: 6.
[Crossref] [Google Scholar]
- Voorhees JM, Barnes ME, Chipps SR, Brown ML (2019) Bioprocessed soybean meal replacement of fish meal in rainbow trout (Oncorhynchus mykiss) diets. Cogent Food Agricult 5: 1579482.
[Crossref] [Google Scholar]
- Mulcahy DM (2003) Surgical implantation of transmitters into fish. ILAR J 44: 295-306.
[Crossref] [Google Scholar] [PubMed]
- Zale AV, Brooke C, Fraser WC (2005) Effects of surgically implanted transmitter weights on growth and swimming stamina of small adult westslope cutthroat trout. Trans Am Fish Soc 134: 653-660.
[Crossref] [Google Scholar]
- Perry RW, Plumb JM, Fielding SD, Adams NS, Rondorf DW (2013) Comparing effects of transmitters within and among populations: Application to swimming performance of juvenile Chinook salmon. Trans Am Fish Soc 142: 901-911.
[Crossref] [Google Scholar]
- Daniels WH, Robinson EH (1986) Protein and energy requirements of juvenile red drum (Sciaenops ocellatus). Aquaculture 53: 243-252.
[Crossref] [Google Scholar]
- Kim JD, Kaushik SJ (1992) Contribution of digestible energy from carbohydrates and estimation of protein/energy requirements for growth of rainbow trout (Oncorhynchus mykiss). Aquaculture 106: 161-169.
[Crossref] [Google Scholar]
- Leao T, Siqueira M, Marcondes S, Franco‐Belussi L, De Oliveira C, et al. (2021) Comparative liver morphology associated with the hepatosomatic index in five neotropical anuran species. Anatom Rec 304: 860-871.
[Crossref] [Google Scholar] [PubMed]
- Yıldız M, Sener E, Timur M (2006) Effect of seasonal change and different commercial feeds on proximate composition of sea bream (Sparus aurata). Turk J Fish Aquat Sci 6: 99-104. [Crossref]
[Google Scholar]
- Jobling M, Koskela J, Savolainen R (1998) Influence of dietary fat level and increased adiposity on growth and fat deposition in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29: 601-607.
[Crossref] [Google Scholar]
- Company R, Calduch-Giner JA, Kaushik S, Perez-Sanchez J (1999) Growth performance and adiposity in gilthead sea bream (Sparus aurata): Risks and benefits of high energy diets. Aquaculture 171: 279-292.
[Crossref] [Google Scholar]
- Igejongbo TF, Esther O (2022) Gut content and viscerosomatic index analysis of family Clariidae in the riverine area of south western Nigeria. East Afric Schol J Agricult Life Sci 5: 53-59.
[Google Scholar]
- Thoreau X, Baras E (1997) Evaluation of surgery procedures for implanting telemetry transmitters into the body cavity of tilapia Oreochromis aureus. Aquat Liv Res 10: 207-211.
[Crossref] [Google Scholar]
- Campbell JM, Carter PA, Wheeler PA, Thorgaard GH (2015) Aggressive behavior, brain size and domestication in clonal rainbow trout lines. Behav Genet 45: 245-254.
[Crossref] [Google Scholar] [PubMed]
- Goncalves-de-Freitas E, Bolognesi MC, Gauy AC, Brandao ML, Giaquinto PC, et al. (2019) Social behavior and welfare in Nile tilapia. Fishes 4: 23.
[Crossref] [Google Scholar]
- Hinshaw JM (2000) Trout farming. Southern Regional Aquaculture Center Publication.
- Liedtke TL, Beeman JW, Gee LP (2012) A standard operating procedure for the surgical implantation of transmitters in juvenile salmonids. US Geological Survey Publications, United States.
[Crossref]
- Barton BA, Peter RE, Paulencu CR (1980) Plasma cortisol levels of fingerling rainbow trout (Salmo gairdneri) at rest and subjected to handling, confinement, transport and stocking. Canad J Fish Aquat Sci 37: 805-811.
[Crossref] [Google Scholar]
- Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann Rev Fish Dis 1: 3-26.
[Crossref] [Google Scholar]
- Conte FS (2004) Stress and the welfare of cultured fish. Appl Anim Behav Sci 86: 205-223.
[Crossref] [Google Scholar]
- Lai JC, Kakuta I, Mok HO, Rummer JL, Randall D (2006) Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J Exp Biol 209: 2734-2738.
[Crossref] [Google Scholar] [PubMed]
- Kientz J, Huysman N, Barnes ME (2021) A comparison of cyanoacrylate to sutures for wound closure following acoustic transmitter insertion in rainbow trout. Aquac Fish 6: 513-518.
[Crossref] [Google Scholar]
- Marty GD, Summerfelt RC (1986) Pathways and mechanisms for expulsion of surgically implanted dummy transmitters from channel catfish. Trans Am Fish Soc 115: 577-589.
[Crossref] [Google Scholar]
- Deters KA, Brown RS, Carter KM, Boyd JW, Eppard MB, et al. (2010) Performance assessment of suture type, water temperature and surgeon skill in juvenile Chinook salmon surgically implanted with acoustic transmitters. Trans Am Fish Soc 139: 888-899.
[Crossref] [Google Scholar]
- Rub AM, Jepsen N, Liedtke TL, Moser ML, Weber ES (2014) Surgical insertion of transmitters and telemetry methods in fisheries research. Am J Vet Res 75: 402-416.
[Crossref] [Google Scholar] [PubMed]
- Suzuki Y, Lida T (1992) Fish granulocytes in the process of inflammation. Ann Rev Fish Dis 2: 149-160.
[Crossref] [Google Scholar]
- Huyben D, Vidakovic A, Sundh H, Sundell K, Kiessling A, et al. (2019) Haematological and intestinal health parameters of rainbow trout are influenced by dietary live yeast and increased water temperature. Fish Shellfish Immunol 89: 525-536.
[Crossref] [Google Scholar] [PubMed]
- Shameena SS, Kumar S, Kumar K, Raman RP (2021) Role of temperature and co-infection in mediating the immune response of goldfish. Microb Pathog 156: 104896.
[Crossref] [Google Scholar] [PubMed]
- Kume M, Takagi J, Dantsuji Y, Ito T, Yamashita Y, et al. (2023) Tagging of age-0 flatfish with acoustic transmitters: Comparison of internal implantation versus external attachment. Environ Biol Fish 106: 2011-2019.
[Crossref] [Google Scholar]
- Mensinger MA, Hawkes JP, Goulette GS, Mortelliti A, Blomberg EJ, et al. Dams facilitate predation during Atlantic salmon (Salmo salar) smolt migration. Can J Fish Aquat Sci 81: 38-51.
[Crossref] [Google Scholar]
Citation: Wagner A, Huysman N, Kientz J, Voorhees JM, Barnes ME (2024) Micro-acoustic Transmitter Implantation Impacts Juvenile Rainbow Trout Oncorhynchus mykiss Growth, Hematocrit and Splenosomatic Index. J Fish Sci, Vol.18 No.6
















