Key words
|
| |
| Psoriasis, Microemulsion, Tea tree oil, Antiinflammatory, transdermal delivery |
| |
Introduction
|
| |
| Microemulsions are optically transparent, low viscosity, and thermodynamically stable dispersions of oil and water stabilized by an interfacial film of a surfactant, usually in combination with a cosurfactant. In pharmaceutics, microemulsions are used as vehicles to deliver many kinds of drugs because of their thermodynamic stability, ease of preparation and good appearance. |
| |
| (1) Formulations based on microemulsions have several interesting characteristics viz. enhanced drug solubilization, good stability, and ease of manufacturing. Although microemulsion can be used to deliver drugs via several routes; the system has been extensively studied as vehicle for topical and transdermal administration. (2) In topical and transdermal formulations, microemulsions have been proved to increase the cutaneous absorption of both lipophilic and hydrophilic API’s when compared to conventional vehicles (emulsions, pure oils, aqueous solutions, etc). (3) Microemulsions (ME) that is spontaneously formed by combining appropriate amounts of a lipophilic and a hydrophilic ingredient, as well as a surfactant and co-surfactant can be used. Due to their special features, microemulsions offer several advantages for pharmaceutical use, such as ease of preparation, long-term stability, high solubilization capacity for hydrophilic and lipophilic drugs, and improved drug delivery. (4) |
| |
| Psoriasis is an inflammatory and proliferate disease of skin that results in rapid turnover of skin cells. The turnover of skin cells can rise up to seven times of the normal rate, leading to thickening of superficial layers of skin. The typical skin change (primary lesion) of psoriasis is a sharply demarcated erythrosquamous plaque; it appears infiltrated and reddened as a clinical correlate of inflammation and scaly as a sign of hyperparakeratosis. (5) The tea tree oil is extracted by steam distillation from the leaves and twigs of the native Australian shrub Melaleuca Alternifolia. The tea tree oil for therapeutic use is clear/pale yellow in appearance with a clean, fresh medicinal aroma. It can alleviate inflammation and may help wound healing. (6) Terpinen-4-ol is the major TTO component and has shown strong antimicrobial and anti-inflammatory properties. (7) In vitro work over the last decade has demonstrated that terpinen-4-ol can inhibit the production of several inflammatory mediators (such as interleukins) by human peripheral blood monocytes. (8) This suggests a mechanism by which TTO may reduce the normal inflammatory response in psoriasis through its action below the dermis. Therefore such a delivery system is desired which can release drug transdermally and show its action. |
| |
| The aim of the present study was to develop and evaluate a novel delivery system such as microemulsion for the topical administration of tea tree oil in psoriasis. Tea tree oil has antiinflammatory effects that could help to treat patients suffering from psoriasis. In this work the ability of a microemulsion system to incorporate and deliver Tea tree oil components across skin has been investigated and the microemulsion is characterized for various parameters like viscosity, droplet size, microstructure, permeation, irritation etc. |
| |
MATERIALS AND METHODS:
|
| |
|
Materials
|
| |
| Tea Tree Oil was purchased from Japan Bottle House, Chawri Bazar. Tween 80 and Isopropyl Alcohol was purchased from Qualigens, Mumbai. Iso propyl myristate was procured from GS Chemical Testing Lab & Allied Industries, Bombay and Glycerol from Merck, Mumbai. All other chemicals used were of AR grade and used without further purification. |
| |
|
Preparation of Microemulsion
|
| |
| A series of microemulsion formulations were prepared using Tea tree oil as the oil phase, Tween 80 as surfactant and Isopropyl alcohol and Isopropyl myristate as cosurfactants. In all the formulations, the amount of Tea tree oil was kept constant at therapeutic levels as high concentrations of Tea tree oil can produce skin irritation. Accurately measured oil was placed in a beaker, surfactant, and cosolvent were added and the components were mixed by gentle stirring on magnetic stirrer and homogenized. (9) Microemulsions were prepared using 5% tea tree oil and total aqueous phase constituting glycerol and water concentration was fixed to 30%. Five microemulsion formulations were prepared i.e. ME-1 to ME-5 in varying concentration of surfactant and cosurfactants mentioned above and were characterized for various parameters. |
| |
|
Characterization of Microemulsion
|
| |
|
Determination of Physical Parameters
|
| |
| Studies were carried out to determine the physical parameters of the microemulsions such as physical appearance, type of emulsion, pH etc. Physical apperance of the microemulsion was analysed visually. The type of emulsion was determined by dilution test. Small amount of microemulsion was placed on a clean glass slide. A drop of water added to the microemulsion and was mixed with the help of glass rod and their transparency was assessed visually. (11) The microemulsion’s pH was determined using (Eutech, New Delhi) pH tutor. The pH meter was calibrated using pH buffer solution and the pH of each microemulsion preparation was determined in triplicate. (12) |
| |
|
Viscosity Measurements
|
| |
| Microemulsions are generally low viscosity systems. The viscosity measurements were performed using Brookfield’s viscometer (Brookfield, USA) at single mode (Spindle C-50). All the measurements were done in triplicate for 60 seconds at a temperature of 23.5°C. (13) |
| |
|
Determination of Droplet size and Polydispersity Index
|
| |
| Droplet sizes of microemulsion were determined by using dynamic light scattering method using Melvern zeta master (UK). Samples were loaded into 1-cm2 cylindrical cuvettes and placed in a thermostated scattering chamber. Light scattering was monitored at a fixed angle of 90? and fixed temperature of 25o C. One ml of formulation was diluted to 10 ml in a test tube and gently mixed and was analysed. (14) Polydispersity is the ratio of standard deviation to mean droplet size, so it indicates the uniformity of droplet size within the formulation. (15) The polydispersity index of the formulation was determined by the same instrument. |
| |
|
Analysis of Drug Content by HPTLC
|
| |
| The terpinen-4-ol content in the formulation was analyzed by dissolving microemulsion in 10ml of ethanol. To ensure complete extraction of terpinen-4- ol, it was sonicated for 15 minutes. The resulting solution was centrifuged and the supernatant was analyzed for terpinen-4-ol content. Terpinen-4-ol was used as the reference standard and the formulation was spotted on the precoated TLC plates and developed using a mixture of toluene and ethyl acetate (85:15) as mobile phase with 2 dimensional developments. 5?l of the supernatant was spotted on the plate and analyzed for terpinen-4-ol content by HPTLC (CAMAG, Switzerland). The following conditions were maintained: |
| |
| Chamber saturation time : 15 mins |
| |
| Temperature : 23-27 °C |
| |
| Migration distance : 80mm |
| |
| TLC plates were dried completely. To visualize the zones, plates were sprayed with an anisaldehyde reagent and heated in oven. The calculations were done on the basis of Area under curve (AUC) by test solution and by standard. (10) |
| |
|
Surface Morphology and Shape
|
| |
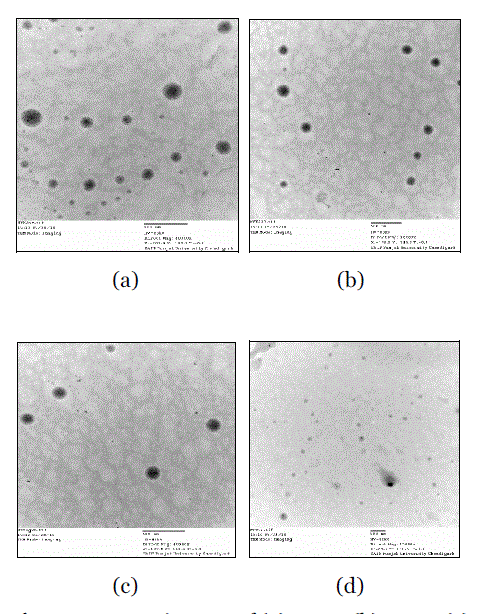
| Transmission Electron Microscopy characterized microstructure of the formulation. Microemulsions were prepared at ambient conditions. A drop of solution was placed on a pure thin bar mesh TEM grid (Philips, CM-10). The drop was blotted with filter paper until reduced to a thin film. The sample was then vitrified by rapidly immersing into liquid ethane near its freezing point. The vitrified specimen was transferred to TEM for imaging and scanning of the specimen produced distinct TEM pictures. (16) |
| |
|
In vitro Skin Diffusion Studies
|
| |
| Excised skin of Wistar rats was left overnight for equilibration in phosphate buffer saline. Phosphate buffer saline pH 7.4 at a temperature of 350C was used as the receptor phase. The excised skin was mounted with stratum corneum on the upper side in modified Franz-type diffusion cells. Microemulsion was spread evenly on the skin of about 3-4 cms. Samples from the receptor phase (10 ml) were removed at 2, 4, 6, 8, 12 and 24 hours and the sample were analyzed for terpinen-4-ol content by HPTLC. (10) |
| |
|
Skin Irritation studies
|
| |
| The skin irritation studies for microemulsion were carried out on Swiss albino mice weighing 25-35 gms. The experiments were carried out after taking due approval from Animal ethical committee. The animals were kept under standard laboratory conditions and housed in polypropylene cages. The animals were divided into four groups. Group I was taken as negative control and Group II served as positive control. Group III and IV were applied with microemulsion formulations. A single dose of the microemulsion was applied to right ear of the mice keeping left as control in Group III and IV. In Group II. 10% Tea tree oil was applied to the right lobe as positive control for a period of 21 days. The development of erythema or skin irritation was visualised after a total period of 21 days. |
| |
|
Stability studies
|
| |
| Accelerated stability is used to assess the shelf life of a product, but the conditions of storage are more harsh (i.e. higher temperature and increased humidity). This is done to provide an estimated shelf life of the product in a shorter time period than ’real time’ stability. The purpose of stability study is not only to characterize the degradation of a drug product but also to establish an expiration-dating period or shelf life applicable to all future batches of drug product. The accelerated stability studies were carried out at 40O C and 75% Relative humidity. The samples of microemulsion were kept for 6 weeks in the stability chamber (Scope Enterprises, New Delhi) and the samples were withdrawn and analysed at a period of 0, 2, 4 and 6 weeks. The samples were analysed for physical stability, droplet size, drug content, pH and viscosity. |
| |
RESULTS AND DISCUSSIONS
|
| |
|
Results of Physical Parameters
|
| |
| The formulated microemulsions ME-1 and ME-2 were found to be translucent whereas the microemulsions ME-3, ME-4 and ME-5 were observed to be clear visually. The dilutability of the microemulsions was assessed to know whether these systems would be diluted with the aqueous phase without separation or not. On the basis of dilution test the microemulsion ME-1 to ME-5 were characterized as oil in water microemulsion (O/W). The measurements were repeated after 15 and 30 days of preparation to analyze any change in pH. The pH of the microemulsions ME-1 to ME-5 is depicted in Table 1. |
| |
|
Viscosity Measurements
|
| |
| For rheological measurement the viscosity of microemulsions, were measured at different shear and constant temperature (23.5°C) using a viscometer (Brookfield Viscometer) for 60 seconds. The mean viscosity of the microemulsions was found to be in the range of 0.51-0.63 PaS as shown in Table 2. |
| |
|
Droplet size and Polydispersity Index
|
| |
| The droplet size distribution graph depicted that maximum number of oil droplets were in the range of 80-90 nm for ME-5. The mean average size of ME-5 the droplets were observed to be 84 nm as depicted in Figure 2. The droplet size distribution graph as shown in Figure was bell shaped graph with even distribution range. As, the higher the polydispersity, the lower the uniformity of the droplet size in the formulation. Therefore, the low polydispersity index of the formulation indicates higher uniformity of droplet size in the formulation, which was found to be in ME-5. |
| |
|
Drug Content in Microemulsion
|
| |
| HPTLC analysis was done in order to determine the content of terpinen-4-ol in the formulation. The content of terpinen-4-ol was found to be maximum in microemulsion ME-5 i.e. 1.68µg in 5 ?l of the sample applied. The results obtained are summarized in Table 4 below. |
| |
|
Surface Morphology and Shape
|
| |
| The TEM micrographs of these formulations are presented in Fig 3. Based on the surface morphology and structure results it was observed that ME-5 showed better results as compared to other microemulsions. In the TEM positive image, the microemulsion appeared dark and the surroundings were bright. Some droplets sizes were measured, as TEM is capable of point-to-point resolution. TEM images revealed and confirmed the lowest droplet size of ME-5. The TEM images of the microemulsion depicted spherical shape and even boundary of the oil particles. |
| |
|
In vitro Skin Diffusion Studies
|
| |
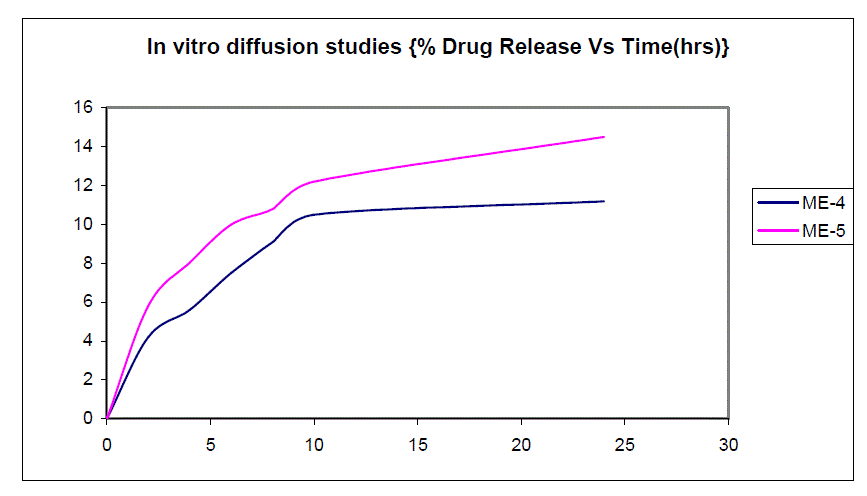
| For in vitro skin diffusion study, excised skin of Wistar rats was taken as animal model. The experiment design was chosen to mimic the normal in use conditions. The release profile of terpinen-4-ol from ME-5 depicted that there was a total of 14.5% release through the skin after 24 hours of application of ME-5 on the skin. This proved the enhance ability of microemulsions to permeate through the skin. Similarly in vitro diffusion studies were performed with ME-4. Figure 4 shows a peak display for ME-4 after 24 hours of application of microemulsion. ME-4 showed 11.2% of drug release from the microemulsion. It was observed that there was enhanced penetration of terpinen-4-ol when tea tree oil is formulated in form of microemulsion. The plateauing effect seen in the penetration profiles was consistent with finite dosing and depletion of TTO components from the application site resulting in lowering of the concentration gradient across the membrane and slowing of the penetration rate. |
| |
|
Skin irritation studies
|
| |
| It was observed that the microemulsion application on Swiss albino mice showed no signs of erythema and skin irritation on Group III and IV i.e. ME-4 and ME-5 even on application for 21 consecutive days. |
| |
| Thus it can be concluded that 10% tea tree oil is unsafe for application on skin and levels up to 5% tea tree oil can be safely applied to the skin as no skin irritation was observed when 5% of tea tree oil was applied in the form of microemulsion. |
| |
|
Stability Studies
|
| |
| The results of stability studies can be used to characterize the relationship between degradation and storage condition. The microemulsions were found be physically stable and no signs of creaming; cracking or phase separation was seen during the accelerated stability studies. This signifies that the microemulsion was physically stable at 40°C / 75% RH. The mean droplet size of the microemulsion was found to increase but not to very significant level. Drug content was found to decrease during the stability studies. This can be attributed to the fact that terpinen-4-ol converts into p-cymene at elevated temperatures. No significant change in the viscosity of the microemulsion was observed. |
| |
CONCLUSION
|
| |
| Novel microemulsion was prepared using 5% tea tree oil with Tween 80 as surfactant and Isopropyl alcohol and Isopropyl myristate as cosurfactants. The formulation is easy to scale up as the procedure is simple and does not involve lengthy procedure and unnecessary use of pharmaceutically unacceptable additives. The utilization of microemulsion systems offers a number of advantages. The advantage of using O/W microemulsion for delivery of essential oils is the possibility that it could retard the volatilisation, oxidation, and degradation of the essential oil during processing and storage. Main point of criticism is the necessity of large amounts of surfactants to form microemulsions. Throughout the literature this problem occurs as most microemulsions contain <40% of surfactants. Considering the potential in enhanced drug uptake and facing the limitations their unique properties make microemulsions a promising vehicle for dermal and transdermal drug delivery. |
| |
Conflict of Interest Declaration
|
| |
| I hereby declare that I have no competing interests. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
| |








