Keywords
Clinical Research; Pharmaceutical market; Clinical trials
Introduction
Clinical Research or clinical investigation is an imperative part of the drug discovery process that involves testing of a new drug in humans to evaluate the potential effects and determine the safety of a drug under investigation. In present scientific era, clinical researches are the mainstay for introducing improved therapies and newer drugs to the market [1].
Asia being the fastest growing pharmaceutical market continue to venture clinical researches following globalization of clinical trials. Approximately 30% of the clinical research activities are being outsourced in Asia by the multinational pharmaceutical companies. The swift shift to Asian clinical research market particularly China, Japan, India, is expected to mount faster than Europe and United States (US) [2]. With over 60% world’s population, the attention toward the Asian countries may be due to huge patient pool with limited access to affordable medicine. Consequently, attributing to the quest of global pharmaceuticals in exploring better avenues to expand their enterprise. The increasing prevalence of diseases such as hypertension, diabetes, and dyslipidemia is the main reason to conduct clinical research trials in Asia [3,4].
Pakistan stands as 10th largest pharmaceutical industry in Asia Pacific with US$1.64bn [5]. The number of diseases prevailing in Pakistan is higher than the average compared to other regions of South Asia. Majority of health infrastructure of Pakistan comprising of hospitals/institutions, dispensaries, health centers and basic units are available in urban areas leaving most of the vulnerable population in rural areas with limited access to treatment. In Pakistan, clinical trials are still in their infancy while its neighbor is emerging as a preferred destination by outsourcing. WHO establish an international registry clinical trials (ICTRP Platform) to make sure that all clinical trials are not only registered but are publicly accessible and easily identifiable). Unfortunately, Pakistan’s trial registration authority is undeveloped and therefore many local researchers seek international registries to publish their research work in international journal of repute. Out of over 60,500 clinical trials conducted around the globe, Pakistan shares only 0.1% despite of having vast patient pool, physicians, available medical centers and low value of rupee. Figure 1A. indicates the number of studies conducted around the globe (region-wise). The WHO international clinical trials registry platform (ICTRP) shows only 311 registered trials of Pakistan dating back to 1999, with almost 29 trials registered in 2013 alone [6], as illustrated in Figure 1B. An exponential growth in clinical trials has been noted from 2005, the activity continued at a steady rate. However, this doesn’t depict the exact clinical activities, the growth in clinical research is encouraging in Pakistan. Registration of a trial in Pakistan goes unrecognized, that is why most global research make the registry while the local trial data is not acquired entirely. The research group in Pakistan has the essential infrastructure, regulatory policies, ethics committees, and patient pool to attract international business. Many research cooperatives, research institutions and other established groups have added research to their clinical program like Medical Research Society Pakistan, Neurology Awareness and Research Foundation, Pakistan Society of Clinical Oncology-Cancer, and Pakistan Society of cardiovascular and thoracic surgeons [7].
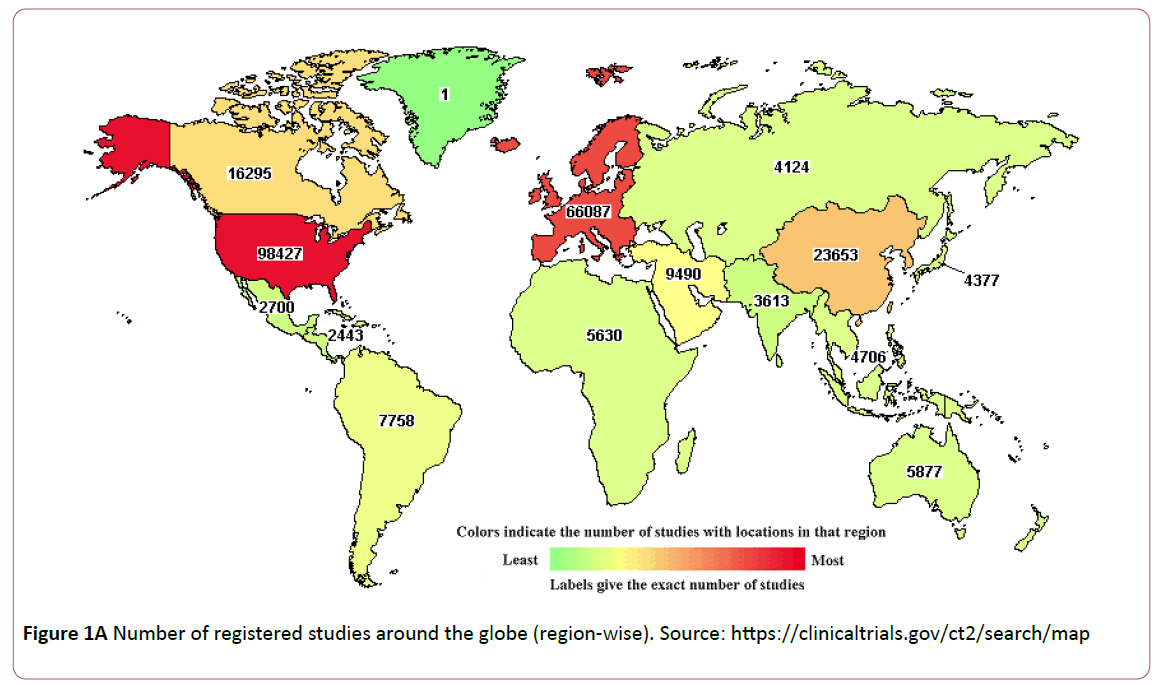
Figure 1A: Number of registered studies around the globe (region-wise). Source: https://clinicaltrials.gov/ct2/search/map
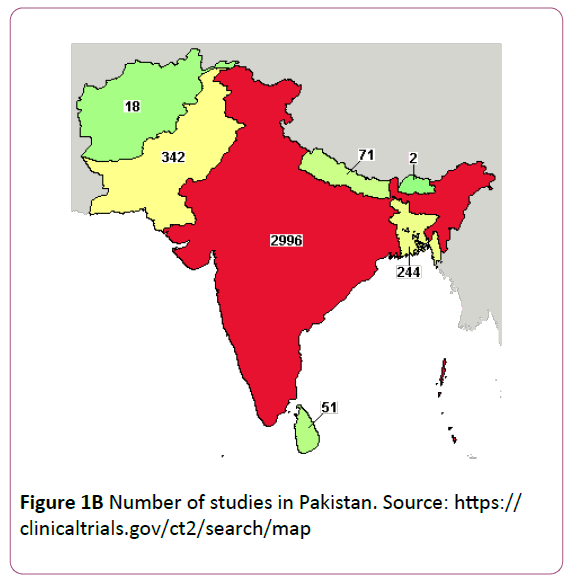
Figure 1B: Number of studies in Pakistan. Source: https:// clinicaltrials.gov/ct2/search/map
Structure of the Pakistan health care system
Health system of Pakistan is divided into public and private sector. While the preference is given to private facilities, about 77% of the healthcare facility visits are mostly private [8]. In Pakistan, the delivery of health services is provincial responsibility and federal government only provide the support and play the coordinating role. According to the statistics of 2014, a total of 13633 health institutions were registered nationwide with approximately 109,960 hospital beds (Table 1). Since urban-rural disparities exists among the health sectors of Pakistan mainly due to insufficient health force and facilities, lady health worker (LHW) program was launched by the government to provide basic primary care. Though only primary health services are accessible to poor, tertiary care is still limited. Government allocate a portion of funds annually but due to current economic development, however, funds have been reduced leading to a decline in quality of health services. Pakistan Economic Survey in Table 1 shows the health care infrastructure of 2012-2013. The health services (private) comprise of physicians, nurses, pharmacists, paramedics, laboratory operators, traditional healers and also inexpert practitioners. For every 1099 patients, only one doctor is available while the requirement of population for every hospital bed is around 1647 [9]. In 2013, from gross domestic product (GDP) Pakistan spent only 1% on health which is inadequate to fulfil the needs of the country [10].
| Number of Private Health Institutions |
73,650 |
| Hospitals |
1096 |
| Rural Health Centers |
650 |
| Basic Health Units |
5527 |
| Dispensaries |
5310 |
Source: Pakistan Bureau of Statistics (2014)
Table 1: HealthCare Infrastructure.
Pakistan diseases profile
Pakistan stands among the countries where cases of preventable diseases still exists. The transmission of wild poliovirus (WPV) [11], tuberculosis [12], and malaria [13] prevails at present in Pakistan. According to Centers for Disease Control and Prevention (CDC), a total of 198 WPV cases were reported in 2011 [14], while preventable disease put Pakistan at threat, non-communicable diseases are also raising vigorously in various cities of the country due to lack of concern of the authorities [15]. In Figure 2 portrays the percentage of deaths per 100,000 due to communicable diseases (38%), non-communicable diseases (50%) and injuries in Pakistan [16] however Figure 3 Illustrates the death rate per year.
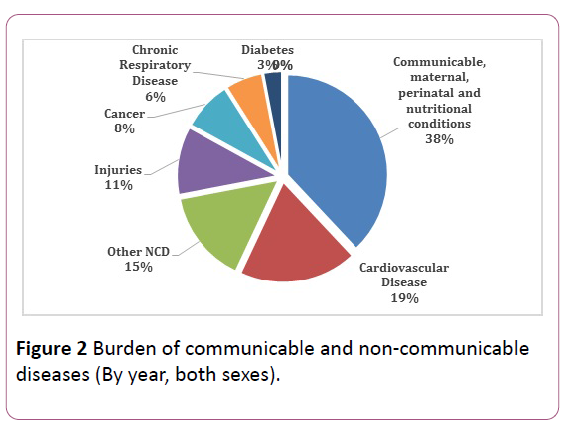
Figure 2: Burden of communicable and non-communicable diseases (By year, both sexes).
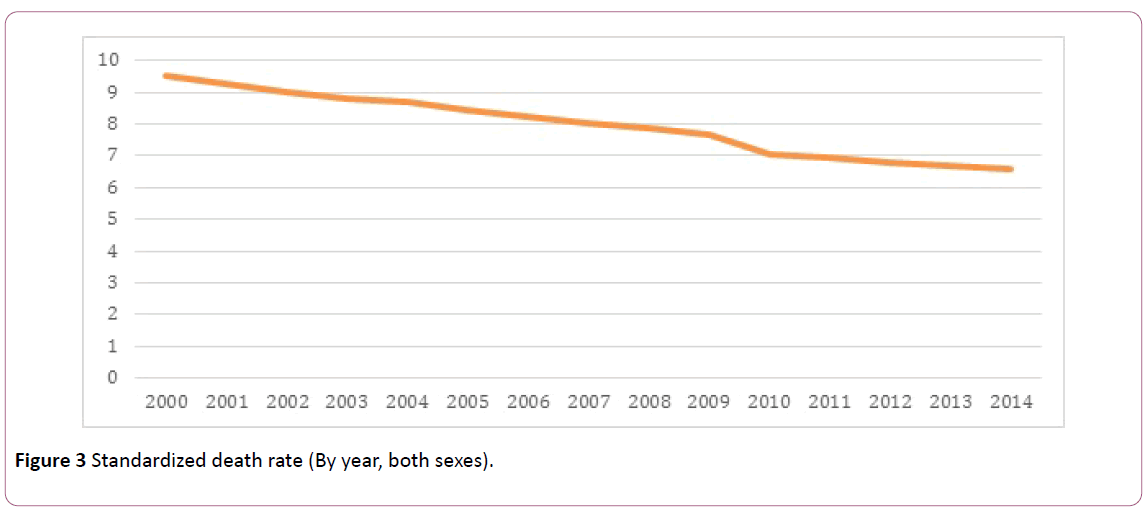
Figure 3: Standardized death rate (By year, both sexes).
Contract research organizations role in clinical research
By 2005, CRO originated countries conducting clinical research had to adopt patent rules in accordance with WHO rules. This helps maintain high standards, such as adherence to protocols set by the industry, better clinical practices and complete documentation. Almost 70% of costs of the research and development (R&D) are used by clinical trials [17]. Pharma of today are under pressure to introduce more new compounds to the market while managing their R&D budgets. According to 2010, the CRO market accounted for nearly $24 billion and in 2015, over 50% growth was noted globally in clinical trials market as shown in Figure 4 [18].
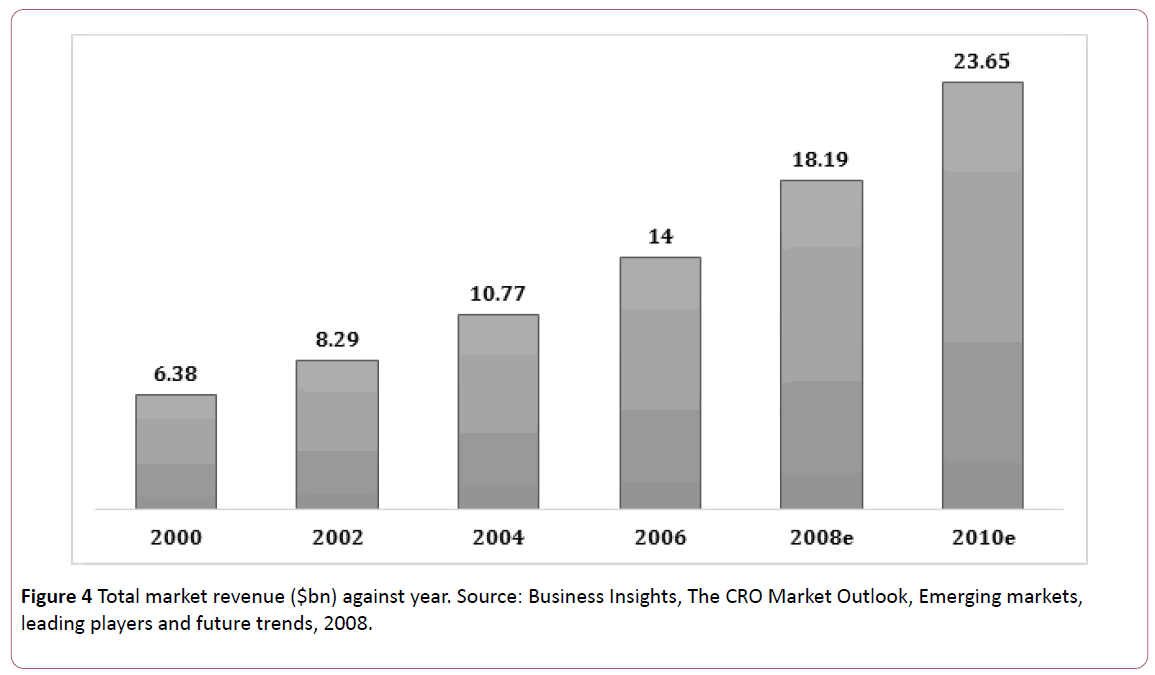
Figure 4: Total market revenue ($bn) against year. Source: Business Insights, The CRO Market Outlook, Emerging markets, leading players and future trends, 2008.
Pakistan has only few active CROs and to promote the business many have established partnerships with international business. Nearly 30 multinationals and over 400 local companies function in Pakistan. Total 80 trials are internationally registered in Pakistan in which 29 are completed and 39 trials are still recruiting [19].
Figure 5 show current clinical trials ongoing in Pakistan, these trials majorly cover the areas of oncology, cardiovascular disorders, infections, metabolic endocrinology, autoimmune diseases and CNS and psychiatry. Multi-national Pharmaceuticals have also been actively participating in clinical trials in Pakistan. In 2004, GlaxoSmithKline became the first to commence the publicly available registry of marketed medicine and trial sponsors [20]. Trade and Development Authority Pakistan (TDAP) recognized the potential of conducting research and organized a conference in 2008 which attracted several international research companies. PharmEvo, as of today, has conducted over 20 clinical trials with 5 registered internationally in the area of cardiovascular diseases, chest infections and blood disorders.
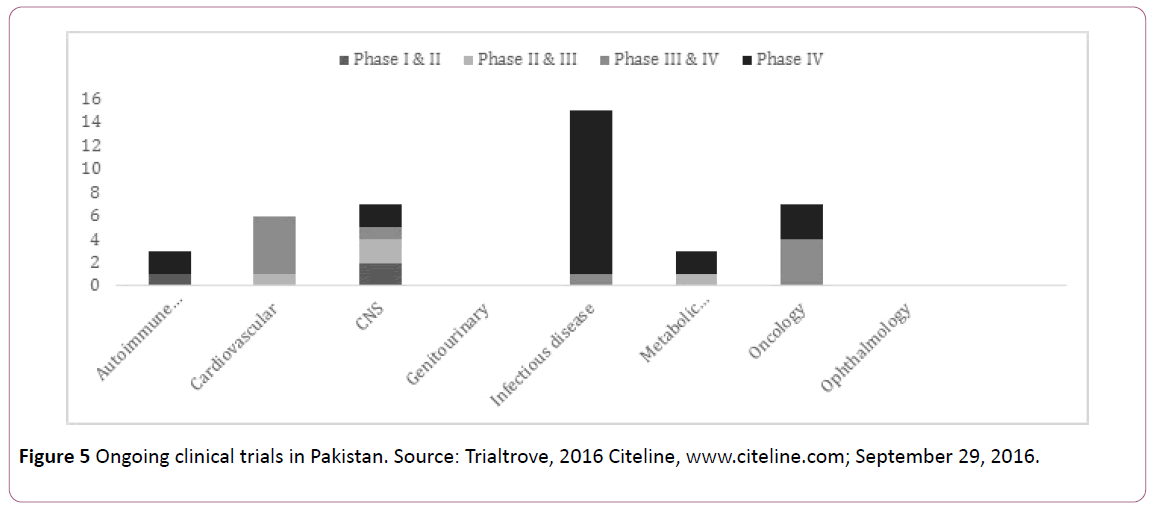
Figure 5: Ongoing clinical trials in Pakistan. Source: Trialtrove, 2016 Citeline, www.citeline.com; September 29, 2016.
Ethical environment and implications
Research Ethics Committees and review boards are formed to ensure compliance with established ethical guidelines and good practices. A gradual increase in a few private healthcare institutions has been noticed over the years to initiate such committees in Pakistan [21]. To obtain research funds, approval by ERC or Institutional Review Boards is still not obligatory, although attention is given to this step to attract medical community and participate in collaborative research projects in United States, the European Union, and organizations like the World Health Organization (WHO).
Ethics or moral behavior is rooted in history since the ancient Egyptian times, it provides the platform for humanity to stand for a compatible existence. The legal and moral guidelines for healthcare professional include proper training, diligence and skills. Malpractice occur when physicians don’t comply with these standards [22]. Evaluating the conflict of interest of a patient and addressing scientific and cultural specificities can be critical in obtaining informed consent.
The ideal situation is when participants of a trial thoroughly read and understand the meaning before signing a consent, but the lack of awareness and illiteracy has limited the practice. Sometimes, informed consent does not exist, and the other times they are inadequate or crude. A sense of crisis exists nationwide that protects the right and welfare of human subject participating in a research. According to a survey, about 40% of patients in Pakistan believe that it is unnecessary or optional to have proper documentation of consent [23]. A strong centralized regulatory body is needed to guide through quality development with extra vigilance in ethical capacity and an understanding of risks involved. A few institutions in Pakistan have their independent setup of ethics board, but the government hospitals are still in need of development. Substantial investment is required to educate professionals, clinicians, and technical staff at all levels about the concept of ethical research.
PharmEvo understands the concept of ethics in research and its implication in Pakistan. The industry is first to initiate the Pakistan Health Research Advisory Board (HealthRAB) that supports the idea to “Develop the Research Ecosystem of Pakistan”. The main objective of this initiative is to provide and support research activities that comply with the ethical guidelines and GCP in Pakistan. Health RAB aims and provision a bridge to international research activities.
Regulatory framework
Just like other developing countries in Asia, the regulatory system in Pakistan is not as established as the other developed countries around the world. The legal established regulations are Drug Act Pakistan, 1976 and Drug (Research) Rules, 1978. Pakistan health authorities apprehends the ethical research and adopted ICH-GCP guidelines, considering it as a gold standard to implement Clinical Good Practice when conducting research. Specific regulations for practicing clinical trials in Pakistan are still under development. The Drug Control Organization under the federal ministry of health (MOH) deals with producer licensing, drug registration, testing, pricing and its trade, whereas the Drug Quality Control Boards that comes under the provincial departments of health (DOH) control market surveillance [19]. Submission of an application is mandatory to import drugs for clinical trials, along with trial protocol, investigational product information, and associated documents to the designated Regulatory authority in Pakistan. The application is first reviewed before issuance of license.
While efforts are being made since the establishment of drug regulatory authority Pakistan (DRAP) for advancement to streamline the process is still demanding. Inadequate resources and unclear policies make the process complicated with inordinate delays. As most of the Institutes have selfregulated bodies, the process does not restrain academia from conducting trial activities that meet international standards and GCP. Due to negligence of authorities and non-availability of national trial registry, journals of Pakistan are experiencing several problems in assenting the articles without UTNs. The regulatory authorities of Pakistan health care, Pakistan medical education and research such as higher education commission (HEC), Pakistan medical and dental council (PMDC), Pakistan medical research council (PMRC) and college of physicians and surgeons Pakistan (CPSP) should take an initiative to inaugurate a standard trial registry platform that meets the criteria of WHO primary registries.
Academic involvement
The academia in Pakistan is very well established and promotes and conduct high quality clinical research. Today, 90 medical schools are recognized by PMDC (the accrediting body). Each year, students especially from recognized universities emigrate for foreign training and only 10% to 15% return to the country, this brain drain has been a huge concern over the years [24]. According to few reports, close to 15,813 Pakistani physicians in 2005 were practicing overseas, representing 17.6% of the total physician’s pool [25].
Despite the alarming emigration of highly trained doctors, large academic centers and hospital still have abundance of qualified and renowned faculty who have been trained internationally and have enough research experience to initiate clinical trials in Pakistan.
Many university hospitals in Pakistan are playing their role actively in scientific discoveries. Such hospitals have their own established clinical units to provide governess and streamline clinical activities. Several institutions made its mark in research education such as Dow University of Health Sciences, Aga Khan University, Sindh Institute of Urology & Transplantation, Shaukat Khanum Memorial Cancer Center offering training workshops and certified clinical research programs.
Clinision, another initiative by PharmEvo that facilitates the development of clinical research in Pakistan and by collaborating with different medical institutes to maximize the capacity of healthcare professionals involved in research.
Although the efforts are vigorous in Pakistan and still underway due to lack of infrastructure and has a long way to establish a position internationally.
Potential market
Pakistan can become an emerging market economy by attracting tremendous opportunities at its doorstep. Since the clinical research has entered Asia, some countries have been able to advance while the unfortunates lag due to poor governance or become a victim of political imbroglios, unfortunately, Pakistan heads the later. The objective of industry is to present leadership in developing research system throughout the country.
The investment policies of Pakistan have been modified overtime to suit investor needs and trends have been consistent with liberalization, privatization, and de-regulation. The capital markets are moderately large and modernized and changes in reforms is underway to develop infrastructure in stock exchange markets.
Conclusion
Due to geopolitical instability and terrorism Pakistan is facing tumultuous times. Despite having a pool of treatment naïve patients, internationally recognized and established institutes, Pakistan is still behind and has not yet made its mark globally while its neighboring country are actively engaging in conducting trials both nationally and internationally. The inaccessibility to rural population is another drawback and major barrier to the market's development. Multinational firms in Pakistan dominates the sector causing a significant trade imbalance in pharmaceuticals, while local companies are focused on generics there are positive signs that domestic industries are ready to move higher the value chain. Pakistan needs to devise better and effective solutions and develop market capacity to streamline its current process.
18735
References
- Waheed S, Siddiqui E (2013) Letter to Editor: The unseen and untold issues of clinical trials and research ethics in Pakistan. Int J Med Biomed Res 2: 161-162.
- Selvarajan S, George M, Kumar SS, Dkhar SA (2013) Clinical trials in India: Where do we stand globally? Perspect clin res 4: 160.
- Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, et al. (2009) Ethical and scientific implications of the globalization of clinical research. N Eng J Med 360: 816-823.
- Zaman K (2011) Review of Pakistan pharmaceutical industry: SWOT analysis. Int J Bus Inf Tech 1.
- (2006) Organization WH. International clinical trials registry platform.
- Irumnaz A, Nair CS (2014) Clinical trials in Pakistan: Opportunities and challenges. Appl Clin Res 1: 23-27.
- Mehboob S (2011) Governance and Militancy in Pakistan’s Khyber Pakhtunkhwa Province. CSIS background paper.
- Ather F, Sherin A (2014) Health system financing in Pakistan: Reviewing resources and opportunities. Khyber Med Uni J 6: 53-55.
- Shaikh BT, Hatcher J (2005) Health seeking behaviour and health service utilization in Pakistan: Challenging the policy makers. J pub health 27: 49-54.
- Moturi EK, Porter KA, Wassilak SG, Tangermann RH, Diop OM, et al. (2014) Progress toward polio eradication-worldwide, 2013-2014. MMWR Morb Mortal Wkly Rep 63: 468-472.
- Ali S, Rabbani F, Siddiqui U, Zaidi A, Sophie A, et al. (2003) Tuberculosis: Do we know enough? A study of patients and their families in an out-patient hospital setting in Karachi, Pakistan. Int J Tuberculosis Lung Dis 7: 1052-1058.
- Bouma M, Dye C, Van der Kaay H (1996) Falciparum malaria and climate change in the Northwest Frontier Province of Pakistan. The Am J Trop Med Hyg.
- Luo HM, Zhang Y, Wang XQ, Yu WZ, Wen N, et al. (2013) Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Eng J Med 369: 1981-1990.
- Pappas G, Akhtar T, Gergen PJ, Hadden WC, Khan AQ (2001) Health status of the Pakistani population: A health profile and comparison with the United States. Am j pub health 91: 93.
- Naseem S, Khattak UK, Ghazanfar H, Irfan A (2016) Prevalence of non-communicable diseases and their risk factors at a semi-urban community, Pakistan. Pan Afr Med J 23.
- Van Huijstee M, Schipper I (2011) Putting contract research organisations on the radar: An exploratory study on outsourcing of Clinical Trials by Pharmaceutical Companies To Contract Research Organisations in Non-Traditional Trial regions.
- Gassmann O, Reepmeyer G, von Zedtwitz M (2008) Leading pharmaceutical innovation: Trends and drivers for growth in the pharmaceutical industry. Springer Science & Business Media.
- Zaidi S, Bigdeli M, Aleem N, Rashidian A (2013) Access to essential medicines in Pakistan: Policy and health systems research concerns. PloS one 8: e63515.
- Rockhold FW, Krall RL (2006) Clinical trials registration. PLoS Med 3: e157.
- Hyder AA, Nadeem S (2001) Health Ethics in Pakistan: A literature review of its present state. J Health, Popul Nutr 6-11.
- Beauchamp TL, Childress JF (1994) Principles of medical ethics. New York: Oxford University Press.
- Gadit A (2008) Migration of doctors: Should we apply the index of happiness? J Pak Med Assoc 58: 342.
- Sheikh A, Naqvi SH, Sheikh K, Naqvi SH, Bandukda MY (2012) Physician migration at its roots: A study on the factors contributing towards a career choice abroad among students at a medical school in Pakistan. Global health 8: 1.












