Keywords
|
| 4-NQO; Chromosome damage; Oxidative stress; Ethanol extract; Mentha spicata |
Abbreviations
|
| 4-NQO: 4?nitroquinoline-1-oxide; EE: Ethanol extract; DMSO: Dimethyl sulfoxide; MnPCEs: Micronucleated Polychromatic erythrocytes; NCEs: Normal chromatic erythrocytes; LPO: Lipid peroxidation; MDA: Malondialdehyde; DTNB: 5,5_-dithiobis (2-nitro benzoic acid); CDNB: 1-chloro 2,4-dinitrobenzene; GPx: Glutathione peroxidase; GST: Glutathiones- transferase; SOD: Superoxide dismutase; CAT: Catalase; GSH: Reduced glutathione; G6PD: Glucose-6-phosphate dehydrogenase; BPO: Benzoxyl peroxide; ANOVA: Analysis of Variance; SNK Test: Student-Neuman-Keuls test; CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals |
Introduction
|
| Oxidative stress is a major factor for the generation of many chronic and degenerative diseases including atherosclerosis, ischemic heart disease, ageing, diabetes, cancer, immunosuppression, neurodegenerative diseases etc. [1]. Many epidemiological and in vitro studies on medicinal plants and vegetables strongly supported that plant constituents with antioxidant activity are capable of exhibiting protective effects against the oxidative stress in biological systems [2]. Hence, research focused on the development of potential plant origin drugs for the elimination of mutagen/carcinogen induced health effects. Many plant extracts have been used as herbal drug for various toxins-induced illnesses in mammals, which have an advantage over the synthetic drug in terms of low or no toxicity at the effective dose [3]. Medicinal plants are also considered to be dietary substance due to their essential bioactive substances like vitamins, carotenoids, and flavonoids, glucoside, organic acids, sterols and some essential oils [4]. |
| Mentha spicata (L.) belongs to the family Lamiaceae containing more than 200 genera and 5600 species [5]. It is also known as spearmint and as “Pudina” in India [6]. The genus Mentha has more than 25 species in which spearmint, peppermint, wild mint, corn mint, curled mint, bergamot, American mint and Korean mint are some well known species and among these spearmint is the most common [7]. The mint smell appears to stimulate the mind to feel for meat. Leaves are consumed in the form of herbal tea [8]. The leaves are used for making juice and chutneys usually in combination with other spices. Boiled leaf extract appears to relieve hiccup, flatulence, giddiness and enhance osteoarthritis symptoms [7,9]. It is also considered as stimulant, carminative and antispasmodic and a remedy for several diseases such as inflammation, fever, bronchitis, infantile troubles, vomiting in pregnancy and hysteria [10]. The plant is also known to have memory enhancing properties [11], antiplatelet [12], pests control [13], cytotoxic [14], chemopreventive [15], antioxidant [16,17] antimicrobial [18,19] and antifungal [20]. Recent reports revealed that M. spicata possessed a potent anti-inflammatory [21], radical scavenging [17] and anticancer properties [22]. Therefore, the present study was to evaluate the modulatory effects of Mentha spicata (Linn.) against 4?nitroquinoline-1-oxide induced chromosome damage and oxidative stress in mice. |
Materials and Methods
|
| Animals: Swiss albino mice were obtained from the King Institute, Chennai, India. Both sex of mice (10-12 weeks old) and weighing 25-30 grams were used. Animals were maintained at the Institute’s animal house under standard environmental conditions (temperature: 22 ± 2°C and 12 h light/dark period). Maintenance of animals was done in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The Institute’s ethical committee approved all experiments. |
| Chemicals used in the study: 4-Nitroquinoline-1-oxide (4- NQO), Malondialdehyde (MDA), Giemsa stain and May-Grunwald stain were purchased from Sigma-Aldrich, USA. Folin phenol reagent, 5,5_-dithiobis (2-nitro benzoic acid) (DTNB), 1-chloro 2,4-dinitrobenzene (CDNB) and reduced glutathione were purchased from SISCO Research Laboratories Pvt. Ltd. (Mumbai, India). All other solvents used in the experiments were of analytical grade. |
| Plant material and extraction method |
| M. spicata L. was commercially purchased and identified at the Center for Advanced Studies in Botany, University of Madras (voucher number-855). Eight hundred grams of shade dried leaf powder was immersed in 4 L of 95% ethanol and left for 24 h under constant stirring and filtered. This was repeated twice with 2 L of 95% ethanol. Thus, a total of 6.5 L filtrate was collected and concentrated by rotary vapor at 40°C. The yield of ethanol extract (EE) was 42 g (5.25%). The extraction was done by the method of Villasenor et al. [23]. |
|
Experimental design
|
| Animals were divided into eight groups, with each group consisting of six mice of either sex. The concentrated ethanol leaf extract was dissolved in 25% DMSO and administered by oral gavage to the mice for 5 consecutive days. Group 1, the control, was administered 25% DMSO. Groups 2, 3, and 4 received doses of 80, 160, and 320 mg/kg bwt extract, respectively. Group 5 was administered 4-NQO alone (7.5 mg/kg bwt intraperitoneally; i.p.). Groups 6, 7, and 8 received the doses of 80, 160, and 320 mg/kg bwt of ethanol extract for 5 days, and after 2 hours, 4-NQO (7. 5 mg/kg bwt) was injected. After 24 hours of 4-NQO treatment, all the animals were sacrificed by cervical dislocation. The mouse bone marrow assay was carried out by the method of Schmid which was previously published [24]. For each animal (experimental/control), 2,500 polychromatic erythrocytes (with or without micronuclei) and a corresponding number of normal chromatic erythrocytes (NCEs) were scored under a light microscope. Mouse liver was immediately excised and store in the physiological saline until use. |
|
Bio-chemical assays estimated by following conventional methods
|
| Homogenate (10%) was prepared in 0.1 M Tris-HCl buffer (pH 7.4), using a Potter-Elvehjem homogenizer with a Teflon pestle. The homogenate was used in bioassays of total protein [25], LPO [26], glutathione peroxidase [27], glutathione-s-transferase (GST) [28], superoxide dismutase (SOD) [29], catalase (CAT) [30], reduced glutathione (GSH) [31], Vitamin E [32] and Vitamin C [33] estimated using conventional procedures. |
|
Statistical analysis
|
| Results were presented as mean ± standard error for six mice of each group. Statistical analyses were performed by one-way ANOVA using SPSS Software Version 12.0. The Student-Neuman-Keuls test (SNK TEST) was applied to assess differences among the groups. Values of P ≤ 0.05 were considered to be significant. |
Results
|
|
Modulated 4-NQO induced genetic damage by M. specata
|
| The results on the frequency of micronucleated polychromatic erythrocytes (MnPCEs) for the various treatment groups are presented in Tables 1 and 2. 4-NQO, enhanced the frequency of MnPCEs by about 4.2 times the control value, 15.78 MnPCEs/2500 PCEs. Irrespective of the dose, treatment with the EE alone had shown no significant effect on the frequency of MnPCEs. However, pre-treatment with EE, significantly reduced the mutation frequency induced by 4-NQO. The reduction was greatest at the highest dose, 320 mg/Kg bwt (by about 69%) and least at the lowest dose, 80 mg/Kg bwt (about 50%). However, the effects by different doses were not consistently significant (P<0.05). |
|
Modulated 4-NQO altered LPO by M. specata
|
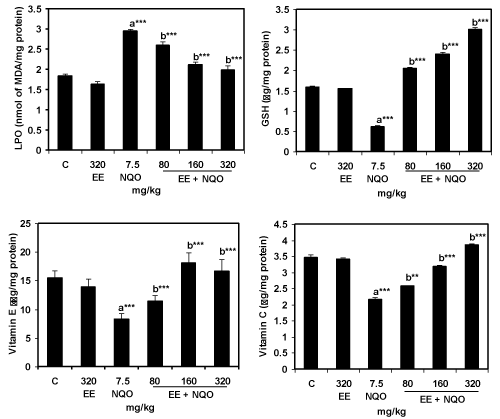
| 4-NQO enhanced the LPO levels by about 60 percent over the control value, 1.84 nmoles of MDA formed/mg protein. EE treatment alone had not shown any effect on the LPO. Pre-treatment with the EE, brought down 4-NQO induced LPO significantly at all the doses tested. At dose 320 mg/Kg bwt, the reduction was effective and comparable with the control value. The reduction of LPO was dose dependent (Figure 1). |
|
Modulated 4-NQO altered enzymatic antioxidants by M. specata
|
| 4-NQO significantly affected the values of enzymatic antioxidants (GPx, GST, SOD and CAT) to an extent of 27 and 41% over their control values (group 1 vs 5, P<0.05, Table 1). Among them, GST was the most affected enzyme. The mutagen affected the enzyme value by about 41% of the control value. Both GPx and SOD were equally affected (~38 and 37%, respectively) by 4-NQO. Treatment with EE alone had shown no effect on any of these enzymes. The levels of all enzymes were restored to their control level with the pretreatment of EE. However, the dose response of recovery varied with the enzymes. The most affected enzyme, GST was restored to the control level only at doses, 160 and 320 mg/Kg bwt. At the lowest dose, 80 mg/Kg bwt, though significant recovery was observed, the enzyme level was not comparable with the control value (P<0.05, Table 1). The recovery of CAT was more dramatic. The lowest dose was not effective (80 mg/Kg bwt; group 5=6, P>0.05). But the dose, 160 mg/Kg bwt itself was most effective. The recovery of SOD was dose dependent. The recovery was effective at the dose of 160 mg/Kg bwt. On the other hand, the recovery of GPx was effective, even at the lowest dose (80 mg/Kg bwt). |
|
Modulated 4-NQO altered non-enzymatic antioxidants by M. specata
|
| Unlike enzymatic antioxidants, the three non-enzymatic antioxidants viz., GSH, Vitamin E and Vitamin C were the most affected (Figure 1). The range of decrease of the non-enzymatic antioxidants was much higher (38-60%), compared to the range of decrease, (27- 41%) observed for the enzymatic antioxidants. Of the three nonenzymatic antioxidants, the GSH decreased to the extent of 60% of the control value, 1.54 μg/mg protein followed by Vitamin E (47%). Their recovery following the pretreatment with EE varied. The GSH recovery was effective even at the lowest dose, 80 mg/kg bwt. In fact, the values of this antioxidant were significantly higher than the control values at doses 160 and 320 mg/Kg bwt. The recovery of Vitamin E was effective only at doses, 160 and 320 mg/Kg bwt. Vitamin C recovery was entirely dose dependent and was effective at the highest dose alone. Thus, LPO induced by 4-NQO was modulated by ethanol extract of M. spicata shade dried leaves by effective restoration of non-enzymatic antioxidants in particular that of GSH. |
Discussion
|
| Reactive oxygen species (ROS) are increasingly produced in animals treated with mutagens [34]. 4-NQO was reported to generate LPO through the formation of superoxide anion and hydrogen peroxide during its metabolism [35]. Thus, overload of ROS leads to oxidative stress by damaging macromolecules [36]. The present study evaluated 4?nitroquinoline-1-oxide (4-NQO) induced chromosome damage and oxidative stress in mice modulated by EE obtained from leaves of M. spicata . 4-NQO enhanced the frequency of MnPCEs by about 4.2 times the control value, 15.78 MnPCEs/2500 PCEs. Treatment with EE effectively decreased the 4-NQO enhanced MnPCE frequency by about 50% to 69%, depending on dose. The maximum amount of reduction i.e., 69% was observed at a dose of 320 mg/Kg bwt. PCE/NCE ratio also significantly decreased by about 0.75 in 4-NQO-alone group when compared to the control value, 1.41. Pretreatment with EE increased the PCE/NCE ratio in the range between 1.11 and 1.25, which was near to the control level. Prior report also demonstrated that Mentha extracts possessed tremendous activity against various mutagens/ carcinogens [37]. |
| LPO increased in biological system due to the overload of oxidative stress and insufficient defense against the mutagen exposure [38]. 4-NQO enhanced LPO by about 60% over the control value, i.e., 1.84 nmoles of MDA formed/mg protein (Figure 1). EE effectively suppressed 4-NOQ enhanced LPO. Reduction was significant although it was not always to the level observed in control groups. Moreover, biological antioxidants primarily restrict the deleterious effects of oxidative stress and their decrease reflects saturation with ROS which in turn may lead to cellular death [39]. 4-NQO affected all in vivo antioxidants measured (Table 1 and Figure 1). The decrease in the enzymatic antioxidants was in the range 27-41% and for non-enzymatic antioxidants in the range 38-60%. The most affected antioxidant was GSH (decreased by ∼ 60%) and the least affected was CAT (decreased by ∼ 28%). EE positively modulated all the measured antioxidants. The magnitude of modulation varied with dose. The modulated values at high doses are either comparable with the controls or even higher than the control values. Mentha extract showed protective effect against gamma radiation exposed to mice by elevating GSH and decreasing LPO [40]. Saleem et al. [15] explored the modulating effect of spearmint extract on benzoxyl peroxide (BPO) altered LPO and antioxidant levels in mice. They observed a significant decrease in LPO and raise in GSH, CAT, G6PD, GPx, GR and GST levels. Similarly, EE of aromatic plants showed protective effects against d-galactose induced oxidative stress in mice [41]. Other experiments supported that the feeding of medicinal plants extracts in rat results in an increase of antioxidant enzyme activity [3]. |
| The mechanism of antioxidant is essential, SOD converts two superoxide anions into an oxygen and hydrogen peroxide molecules. CAT breaks down the H2O2 into oxygen and water. Alternatively, GPx detoxifies the H2O2 and hydroxyl radicals. The latter is more important than the catalase in removing H2O2. GPx also has the ability to react with lipid peroxides [42]. The mutagen 4-NQO can be detoxified by GST with GSH conjugation to give 4 (glutathione-s-yl) quinoline-1- oxides and protect cells from the carcinogenic agents [35]. The present study also revealed that the non-enzymatic antioxidant levels were better than control values (Figure 1). The activities of non-enzymatic antioxidants viz., Vitamin E, Vitamin C and GSH were consistent in metabolisation of mutagens [43]. Vitamin E scavenges the peroxyl and alkoxyl radicals by donating one electron and in turn become tocopheroxyl radical. Vitamin C recycles these radicals back to Vitamin E [44]. The oxidized ascorbic acid (dehydroascorbate) is reduced to ascorbic acid by GSH. Thus, GSH is the primary antioxidant in defense against various environmental toxic chemicals [45]. The present results exhibited that GSH is effective even at the lowest dose, 80 mg/kg bwt and their values were significantly higher than the control. Vitamin E was effective only at doses, 160 mg/kg bwt whereas vitamin C was entirely dose dependent. Yao et al. [46] also reported that Ginkgo biloba extract reduced LPO by enhancing GSH activity in oxidative stress induced rat liver by ethanol. |
| It is well know that M. spicata supported can be used as natural antioxidant because it various biological properties. Since from ancient, it is commonly used edible and medicinal plants in India. Based on the scientific knowledge and the present work clearly revealed the 4-NQO induced chromosome damage with oxidative stress in mice was effectively modulated by ethanol extract of M. spicata leaves due to the presence of synergetic secondary metabolites. |
Acknowledgments
|
| This work funded by University Grant Commission (UGC) was gratefully acknowledged. |
Conflict of Interest
|
| The authors declare that there was no conflict of interest in this current publication. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
| Figure 1 |
|







