Rajasekaran S1*, Pradeepa Prasad1 and Gopal Krishna Rao2
1Department of Pharmaceutical Chemistry, Ikon Pharmacy College, Bheemanahalli, Bengaluru, India
2Goa College of Pharmacy, Panaji, Goa, India
*Corresponding Author:
- Dr. S. Rajasekaran
Department of Pharmaceutical Chemistry
Ikon Pharmacy College
Bheemanahalli
Bengaluru
India
Tel: + (91) 80 22234619
E-mail: rajasekaranpharm@gmail.com
Received Date: June 29, 2020; Accepted Date: July 24, 2020; Published Date: July 31, 2020
Citation: Rajasekaran S, Prasad P, Gopal Krishna R (2020) Molecular Properties and Bio-Activity Score of 2{[2-(4-chlorophenyl)-4- oxoquinazolin-3(4H)-yl]amino}-N-(substitutedphenyl) acetamides. Int J Drug Dev & Res Vol.12 No.3: 153
Keywords
{[2-(4-chlorophenyl)-4-oxoquinazolin-3(4H)- yl]amino}-N-(substitutedphenyl) acetamides; Molecular properties; Bioactivity score
Introduction
The ever growing resistance to antibiotics leads to continuous screening for new biologically effective compounds of either natural or synthetic origin. Quinazoline derivatives are extensively used in pharmaceutical industry, medicine and in agriculture for their wide scope of biological activity [1]. Quinazolinone analogs have been reported for various biological activities such as anti-inflammatory [2], antimicrobial [3], antioxidant [4], anticancer [5] and antihypertensive activities [6]. In the drug discovery study the development of new molecule depends on various parameters and one such is `the rule of 5' that predicts absorption or permeation. The other descriptors include H-bond donors, H-bond acceptors, molecular weight and the calculated Log P (CLogP) value.
The present investigation is to evaluate the molecular properties and the bioactivity score of 2{[2-(4-chlorophenyl)-4- oxoquinazolin-3(4H)-yl]amino}-N-(substitutedphenyl) acetamides (4a-p) that has been reported earlier [7].
Materials and Methods
The molecular structure of 2{[2-(4-chlorophenyl)-4- oxoquinazolin-3(4H)-yl]amino}-N-(substitutedphenyl) acetamides (Figure 1) were drawn using online molinspiration software (www.molinspiration.com) for calculation of molecular properties (Log P, Total polar surface area, number of hydrogen bond donors and acceptors, molecular weight, number of atoms, number of rotatable bonds etc.) and prediction of bioactivity score for drug targets (GPCR ligands, kinase inhibitors, ion channel modulators, enzymes and nuclear receptors).
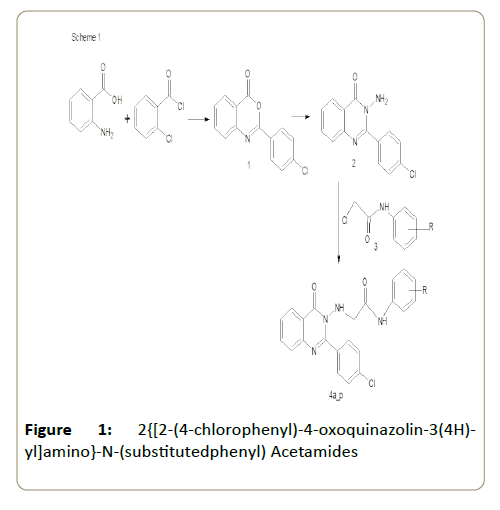
Figure 1: 2{[2-(4-chlorophenyl)-4-oxoquinazolin-3(4H)- yl]amino}-N-(substitutedphenyl) Acetamides
Molinspiration software
Molinspiration software was used to obtain parameter such as MiLogP, TPSA, and drug likeness. Log P measure molecular hydrophobicity that affects drug absorption, bioavailability, drug-receptor interactions, metabolism of molecules, as well as their toxicity. Molecular Polar Surface Area (TPSA) are calculated based as a sum of fragment contributions of O- and N- centered polar fragments and related to the hydrogen bonding potential of a molecule. TPSA is a very good predictor of drug transport properties such as intestinal absorption, bioavailability, blood brain barrier penetration etc. The molecular properties and structure features of a drug can be checked by drug likeness data of a molecule. The calculated value for the drug likeness score and the various parameters of the all the acetamide derivatives were given in Table 1 and the bioactivity scores in Table 2.
| Comp code |
R |
miLogP |
TPSA |
N-atoms |
N-ON |
N-OHNH |
N viol |
N rotb |
volume |
MW |
| 4a |
H |
4.78 |
76.02 |
29 |
6 |
2 |
0 |
5 |
345.17 |
404.86 |
| 4b |
2-CH3 |
5.18 |
76.02 |
30 |
6 |
2 |
1 |
5 |
361.73 |
418.88 |
| 4c |
3-CH3 |
5.2 |
76.02 |
30 |
6 |
2 |
1 |
5 |
361.73 |
418.88 |
| 4d |
4-CH3 |
5.22 |
76.02 |
30 |
6 |
2 |
1 |
5 |
361.73 |
418.88 |
| 4e |
2-Cl |
5.41 |
76.02 |
30 |
6 |
2 |
1 |
5 |
358.71 |
439.3 |
| 4f |
3-Cl |
5.43 |
76.02 |
30 |
6 |
2 |
1 |
5 |
358.71 |
439.3 |
| 4g |
4-Cl |
5.45 |
76.02 |
30 |
6 |
2 |
1 |
5 |
358.71 |
439.3 |
| 4h |
2-NO2 |
4.69 |
121.85 |
32 |
9 |
2 |
0 |
6 |
368.51 |
449.85 |
| 4i |
3-NO2 |
4.71 |
121.85 |
32 |
9 |
2 |
0 |
6 |
368.51 |
449.85 |
| 4j |
4-NO2 |
4.74 |
121.85 |
32 |
9 |
2 |
0 |
6 |
368.51 |
449.85 |
| 4k |
2-Br |
5.54 |
76.02 |
30 |
6 |
2 |
1 |
5 |
363.06 |
483.75 |
| 4l |
3-Br |
5.56 |
76.02 |
30 |
6 |
2 |
1 |
5 |
363.06 |
483.75 |
| 4m |
4-Br |
5.59 |
76.02 |
30 |
6 |
2 |
1 |
5 |
363.06 |
483.75 |
| 4n |
2-OCH3 |
4.79 |
85.26 |
31 |
7 |
2 |
0 |
6 |
370.72 |
434.88 |
| 4o |
3-OCH3 |
4.81 |
85.26 |
31 |
7 |
2 |
0 |
6 |
370.72 |
434.88 |
| 4p |
4-OCH3 |
4.86 |
85.26 |
31 |
7 |
2 |
0 |
6 |
370.72 |
434.88 |
Table 1: Drug likeness score for the compounds
| Comp code |
R |
GPCR ligand |
Ion channel modulator |
Kinase inhibitor |
Nuclear receptor ligand |
Protease inhibitor |
Enzyme inhibitor |
| 4a |
H |
-0.19 |
-0.5 |
-0.11 |
-0.53 |
-0.37 |
-0.17 |
| 4b |
2-CH3 |
-0.24 |
-0.54 |
-0.14 |
-0.52 |
-0.43 |
-0.22 |
| 4c |
3-CH3 |
-0.23 |
-0.56 |
-0.14 |
-0.54 |
-0.42 |
-0.23 |
| 4d |
4-CH3 |
-0.22 |
-0.55 |
-0.15 |
-0.54 |
-0.41 |
-0.21 |
| 4e |
2-Cl |
-0.21 |
-0.49 |
-0.09 |
-0.55 |
-0.41 |
-0.19 |
| 4f |
3-Cl |
-0.2 |
-0.49 |
-0.1 |
-0.52 |
-0.39 |
-0.18 |
| 4g |
4-Cl |
-0.19 |
-0.48 |
-0.1 |
-0.51 |
-0.36 |
-0.16 |
| 4h |
2-NO2 |
-0.3 |
-0.54 |
-0.25 |
-0.68 |
-0.49 |
-0.23 |
| 4i |
3-NO2 |
-0.6 |
-0.51 |
-0.21 |
-0.6 |
-0.46 |
-0.24 |
| 4j |
4-NO2 |
-0.29 |
-0.49 |
-0.22 |
-0.56 |
-0.45 |
-0.23 |
| 4k |
2-Br |
-0.28 |
-0.58 |
-0.2 |
-0.7 |
-0.49 |
-0.23 |
| 4l |
3-Br |
-0.29 |
-0.55 |
-0.12 |
-0.65 |
-0.47 |
-0.24 |
| 4m |
4-Br |
-0.27 |
-0.55 |
-0.14 |
-0.61 |
-0.46 |
-0.22 |
| 4n |
2-OCH3 |
-0.23 |
-0.56 |
-0.13 |
-0.55 |
-0.45 |
-0.22 |
| 4o |
3-OCH3 |
-0.23 |
-0.54 |
-0.13 |
-0.53 |
-0.41 |
-0.21 |
| 4p |
4-OCH3 |
-0.22 |
-0.53 |
-0.13 |
-0.51 |
-0.4 |
-0.2 |
Table 2: Bioactivity score of the compounds
Results and Discussion
The 2{[2-(4-chlorophenyl)-4-oxoquinazolin-3(4H)-yl]amino}- N-(substitutedphenyl) acetamides (4a-p) obeyed the Lipinski’s rule and showed good drug likeness score (Table 1). MiLog P values were found to be below 5 in most of the compounds, however it was higher in the methyl and chloro analogs which indicated good permeability of these compounds. All the derivatives were found to have TPSA in the range of 76.02 to 121.85 (well below 160) and their molecular weights less than 500. Number of hydrogen bond donors (<5) and hydrogen bond acceptors (<7) were found to be within Lipinski’s limit i.e., less than 5 and 10 respectively. All the above compounds were flexible (< 7 rotatable bonds) and found to have n violations =0-1.
Bioactivity score of the compounds
The bioactivity scores of the sixteen acetamide derivatives selected for the calculation on the basis of GPCR ligand, ion channel modulator, nuclear receptor ligand, kinase inhibitor, protease inhibitor, enzyme inhibitor given in Table 2 showed the following observations as per the rule. These scores for organic molecules can be interpreted as active (bioactivity score > 0), moderately active (bioactivity score: -5.0-0.0) and inactive (bioactivity score < -5.0) [8]. All the 2{[2-(4- chlorophenyl)-4-oxoquinazolin-3(4H)-yl]amino}-N- (substitutedphenyl)acetamide derivatives were found to be moderately bioactive (<0) towards all the enzymes considered for the study. However, all the molecules exhibited better activity towards kinase inhibitor compared to other enzymes.
Conclusion
Among the sixteen derivatives though few of them showed higher miLop value all other derivatives obeyed Lipinski rule and the compounds have been found to possess moderate activity towards all the enzymes considered for study, hence the parameters evaluated in this study shall provide an interesting value for the design of novel quinazolinone molecules as enzyme inhibitors.
29427
References
- Selvam P, Babu K, Padmaraj R, Persoon L, Clercq ED (2008) Synthesis, antiviral and cytotoxic activities of some novel 2-Phenyl-3-Disubstituted Quinazolin-4(3H)-ones. African J Pharm and Pharmacol 2:110-115.
- Maggio B, Daidone G, Raffa D, Plescia S, Mantione L, et al, (2001) Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted 3, 4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates. Eur J Med Chem 36: 737-742.
- Sahu SK, Md.Afzal A, Banerjee M, Acharrya S, Beheraam CC, et al, (2008) Synthesis, Characterization and Biological Activity of 2-Methyl-3- aminoquinazolin-4(3H)-ones Schiff Bases. J Braz Chem Soc 19:963-970.
- Melvin JY, Jefferson RM, Richard DT, Peter PRH, Lee AP, et al, Structurally novel antiarrhythmic /antioxidant quinazolines. Bioorg & Med Chem Lett 2:1121-l 126.
- Petr K, Dirk R, Ulrich J (2006) Improved Synthesis of Substituted 6,7-Dihydroxy-4-quinazolineamines: Tandutinib, Erlotinib and Gefitinib. Molecules 11:286-297.
- Alagarsamy V, Pathak US (2007) Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3H-[1,2,4]triazolo[5,1-b]quinazolin-9-ones. Bioorg & Med Chem 15:3457-3462.
- Rajasekaran S, Rao GK, Sanjay PN, Qaseem A, Jasmine (2009) A Brief Review on Thia (Oxa) Diazole Derivatives As Pesticides A. Ind J Het Chem 19:191-192.
- Singh S, Gupta AK, Verma A (2013) Molecular properties and bioactivity score of Aloe vera antioxidant compounds-in order to lead finding. Res J Pharm Biol Chem Sci 4:876-881.







