Key words
|
| |
| Natural Polymers, Transdermal drug delivery, Biodegradable polymers, Control release. |
| |
Introduction
|
| |
| The Polymers are used to control the drug release rate from the formulations [1]. Extensive applications of polymers in drug delivery have been realized because polymers offer unique properties which so far have not been attained by any other materials. Various natural gums and mucilage’s have been examined as polymers for control and sustained drug release, in the last few decades [2]. Natural polymers remain attractive primarily because they are commercial, readily available, capable of multitude of chemical modifications, potentially degradable and compatible due to their origin. |
| |
| A transdermal drug delivery system is a device that is made of one or more types of polymers embedded with drug(s) to deliver the embedded drug through the skin over a controlled period of time [3].In this case, the primary mode of action of the product is controlled drug release and hence it becomes extremely crucial to understand and select materials and techniques that would make it possible to control the drug release [4]. Controlled delivery of drug from the device to the biological system can be achieved by various means. Controlled drug release can also be achieved by embedding the drug onto a polymeric material and then releasing the drug in a predesigned controlled manner from the polymer into the blood stream. This is achieved by the use of transdermal drug delivery system that is made of polymeric materials. |
| |
| Every layer in the transdermal drug delivery system requires properties specific for that layer only. The type of polymer is chosen according to the desired properties for that specific layer. |
| |
| range of materials have been employed to control the release of drugs and other active agents. The earliest of these polymers were originally intended for other, non-biological uses, and were selected because of their desirable physical properties, for example: |
| |
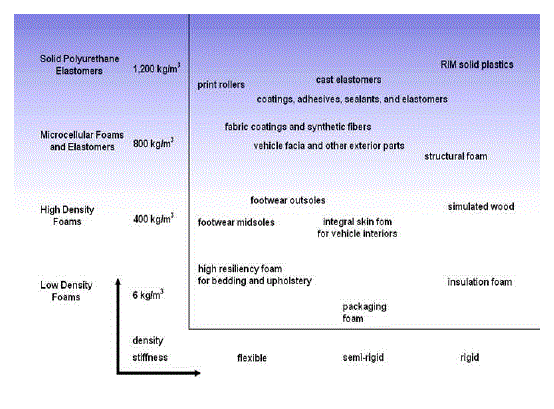
| • Poly (urethanes) : For elasticity and coatings (Shown in Figure 1) |
| |
| • Poly (siloxanes) : Silicones for insulating ability |
| |
| • Poly (methyl methacrylate): For physical strength and transparency. It has a good degree of compatibility with human tissue and can be used to create microfluidiclab-on-achip devices. |
| |
| • Poly (vinyl alcohol): For hydrophilicity and strength. Used for Feminine hygiene and adult incontinence products as a biodegradable plastic backing sheet. |
| |
| • Poly (vinyl pyrrolidone): For suspension capabilities. Mostly used as adhesives. |
| |
| To be successfully used in controlled drug delivery formulations, a material must be chemically inert and free of leachable impurities [5]. It must also have an appropriate physical structure, with minimal undesired aging, and be readily processable. Some of the materials that are currently being used or studied for controlled drug delivery include |
| |
| • Poly(2-hydroxy ethyl methacrylate). |
| |
| • Poly(N-vinyl pyrrolidone). |
| |
| • Poly(methyl methacrylate). |
| |
| • Poly(vinyl alcohol). |
| |
| • Poly(acrylic acid). |
| |
| • Polyacrylamide. |
| |
| • Poly(ethylene-co-vinyl acetate). |
| |
| • Poly(ethylene glycol) |
| |
| • Poly(methacrylic acid). |
| |
| However, in recent years additional polymers designed primarily for medical applications have entered the arena of controlled release. Many of these materials are designed to degrade within the body, among them |
| |
| • Polylactides (PLA). |
| |
| • Polyglycolides (PGA). |
| |
| • Poly(lactide-co-glycolides) (PLGA). |
| |
| • Polyanhydrides. |
| |
| • Polyorthoesters. |
| |
| Originally, polylactides and polyglycolides were used as absorbable suture material, and it was a natural step to work with these polymers in controlled drug delivery systems [6]. The greatest advantage of these degradable polymers is that they are broken down into biologically acceptable molecules that are metabolized and removed from the body via normal metabolic pathways [7]. However, biodegradable materials do produce degradation by-products that must be tolerated with little or no adverse reactions within the biological environment. |
| |
| The other property which is of utmost importance is that of biocompatibility. The polymer that are considered to have properties desirable for transdermal drug delivery system must also be biocompatible since the application of a transdermal patch onto the surface of the skin can be for several days[8]. The other aspect of compatibility is that of chemical compatibility, the drug that is to be embedded in the polymer must be chemically compatible and must not react with the polymer. With the active transdermal systems, it also becomes important that techniques and polymeric materials used for penetrating stratum corneum do not react with the drug that is to be administered [9]. |
| |
| Additionally, a polymer to be used in a transdermal system must satisfy some of the following criteria: |
| |
| • Polymer must be stable, non-reactive with the drug, easily manufactured and fabricated |
| |
| • In expensive and biodegradable |
| |
| • Molecular weight, glass transition temperature and chemical functionality of the polymer should be such that the specific drug diffuses properly and gets released through it. |
| |
| • Polymer and its degradation products must be non- toxic or non – antagonistic to the host. |
| |
| • Polymer mechanical properties should not deteriorate excessively when large amounts of active agent are incorporated into it. |
| |
|
Controlled-Release Mechanisms
|
| |
| Polymers are macromolecules having very large chains, contain a variety of functional groups, can be blended with other low- and high–molecular-weight materials, and can be tailored for any applications. When the drug is delivered to the site of action by using polymer based drug delivery approaches the safety and bio compatibility is questionable [10]. The characterization of biocompatible polymers is more focused in the field of formulation development and drug delivery approaches etc. |
| |
| The biodegradable polymers have properties of degrading in biological fluids with progressive release of dissolved or dispersed drug. There is various novel drug delivery approaches are developed in the pipeline of polymer based drug delivery approaches [11]. The bio-safety and biocompatibility are the important characteristics needed for the use of polymers in the field of pharmaceutical formulation and in novel drug delivery approaches [12]. Regular research is going on in field of use of natural occurring biocompatible polymeric material in designing of topical dosage form for controlled release administration. |
| |
| Controlled-release methodologies can be classified on the basis of the mechanism that controls the release of the active agent from the delivery system: polymer erosion, diffusion, swelling followed by diffusion or degradation. Any of these mechanisms may occur in a given release system. |
| |
| Polymer erosion: The choice of a particular erosion mechanism is dictated by the specific application. The various polymer erosion mechanisms are of 3 basic types. |
| |
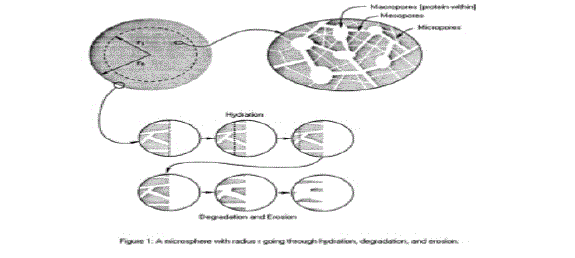
| • Type I erosion involves hydrolysis of hydrogels and these are useful in the controlled release of macromolecules entangled within their network structure(See Figure 2). |
| |
| • Type II erosion involves solubilization of water-insoluble polymers by reactions involving groups pendant from the polymer backbone. Of particular interest are polymers that solubilize by ionization of carboxylic acid groups, and the utilization of those systems is described. |
| |
| • Type III erosion involves cleavage of hydrolytically labile bonds within the polymer backbone and four distinct polymer systems within this category are under development. One system involves the diffusion of drugs from a reservoir through a bioerodible membrane, another system utilizes microcapsules, a third system utilizes monolithic devices, and the fourth system utilizes drugs chemically bound to a bioerodible polymer. |
| |
| In addition, there are 2 mechanisms of polymer release from bio erodible polymers: one approach involves surrounding the drug core with a ratecontrolling bio erodible membrane, while the other involves dispersing the drug within a polymer to form a bio erodible monolithic device [13]. The use of biodegradable systems for the sustained release of fertility-regulating agents is based on type III erosion. Polymer erosion tends to lead drug release, and there is some indication that drug release from the implant is controlled by rate of solubilization of the highly water-insoluble steroid [14]. |
| |
| Diffusion: Diffusion occurs when a drug or other active agent passes through the polymer that forms the controlled-release device [15]. The diffusion can occur on a macroscopic scale as through pores in the polymer matrix or on a molecular level, by passing between polymer chains. For the diffusion-controlled systems, the drug delivery device is fundamentally stable in the biological environment and does not change its size either through swelling or degradation. In these systems, the combinations of polymer matrices and bioactive agents chosen must allow for the drug to diffuse through the pores or macromolecular structure of the polymer upon introduction of the delivery system into the biological environment without inducing any change in the polymer itself [16]. |
| |
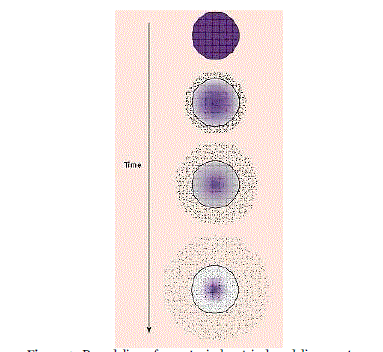
| In Figure 3, a polymer and active agent have been mixed to form a homogeneous system, also referred to as a matrix system. Diffusion occurs when the drug passes from the polymer matrix into the external environment. As the release continues, its rate normally decreases with this type of system, since the active agent has a progressively longer distance to travel and therefore requires a longer diffusion time to release. |
| |
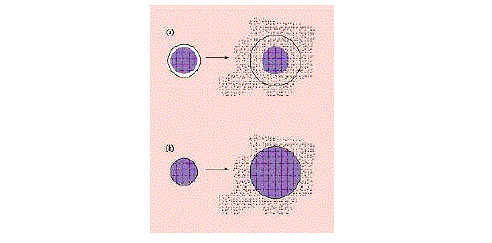
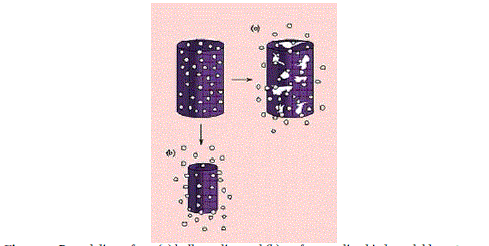
| For the reservoir systems shown in Figures 4a and 4b, the drug delivery rate can remain fairly constant. In this design, a reservoir—whether solid drug, dilute solution, or highly concentrated drug solution within a polymer matrix—is surrounded by a film or membrane of a rate-controlling material. The only structure effectively limiting the release of the drug is the polymer layer surrounding the reservoir. Since this polymer coating is essentially uniform and of a nonchanging thickness, the diffusion rate of the active agent can be kept fairly stable throughout the lifetime of the delivery system. The system shown in Figure 3a is representative of an implantable or oral reservoir delivery system, whereas the system shown in Figure 3b illustrates a transdermal drug delivery system in which only one side of the device will actually be delivering the drug. |
| |
| Swelling-controlled release systems: They are initially dry and, when placed in the body will absorb water or other body fluids and swell. The swelling increases the aqueous solvent content within the formulation as well as the polymer mesh size, enabling the drug to diffuse through the swollen network into the external environment. Examples of these types of devices are shown in Figures 5a and 5b for reservoir and matrix systems, respectively. Most of the materials used in swelling-controlled release systems are based on hydrogels, which are polymers that will swell without dissolving when placed in water or other biological fluids. These hydrogels can absorb a great deal of fluid and, at equilibrium, typically comprise 60–90% fluid and only 10–30% polymer. |
| |
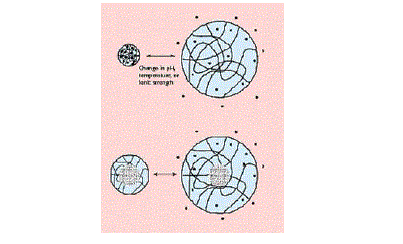
| One of the most remarkable, and useful, features of a polymer's swelling ability manifests itself when that swelling can be triggered by a change in the environment surrounding the delivery system. Depending upon the polymer, the environmental change can involve pH, temperature, or ionic strength, and the system can either shrink or swell upon a change in any of these environmental factors. The diagrams in Figure 6 illustrate the basic changes in structure of these sensitive systems. As many of the potentially most useful pH-sensitive polymers swell at high pH values and collapse at low pH values, the triggered drug delivery occurs upon an increase in the pH of the environment [17]. |
| |
| Degradation: Biodegradable polymer degrades within the body as a result of natural biological processes, eliminating the need to remove a drug delivery system after release of the active agent has been completed. Most biodegradable polymers are designed to degrade as a result of hydrolysis of the polymer chains into biologically acceptable, and progressively smaller, compounds [18]. Degradation may take place through bulk hydrolysis, in which the polymer degrades in a fairly uniform manner throughout the matrix, as shown schematically in Figure 7a. For some degradable polymers, most notably the polyanhydrides and polyorthoesters, the degradation occurs only at the surface of the polymer, resulting in a release rate that is proportional to the surface area of the drug delivery system (see Figure 7b). |
| |
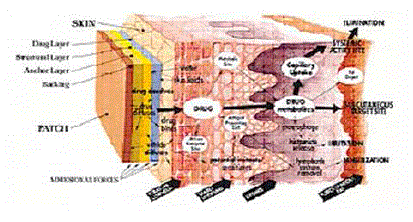
| Once the active agent has been released into the external environment, one might assume that any structural control over drug delivery has been relinquished. However, this is not always the case. For transdermal drug delivery, the penetration of the drug through the skin constitutes an additional series of diffusional and active transport steps, as shown schematically in Figure 8. |
| |
|
Biodegradability of Polymers
|
| |
| Indeed, the term biodegradable plastics normally refer to an attack by microorganisms on nonwatersoluble polymer-based materials (plastics). This implies that the biodegradation of plastics is usually a heterogeneous process. Because of a lack of watersolubility and the size of the polymer molecules, microorganisms are unable to transport the polymeric material directly into the cells where most biochemical processes take place; rather, they must first excrete extracellular enzymes which depolymerize the polymers outside the cells. As a consequence, if the molar mass of the polymers can be sufficiently reduced to generate water-soluble intermediates, these can be transported into the microorganisms and fed into the appropriate metabolic pathway(s).As a result, the end-products of these metabolic processes include water, carbon dioxide and methane (in the case of anaerobic degradation), together with a new biomass. The extracellular enzymes are too large to penetrate deeply into the polymer material, and so act only on the polymer surface; consequently, the biodegradation of plastics is usually a surface erosion process. |
| |
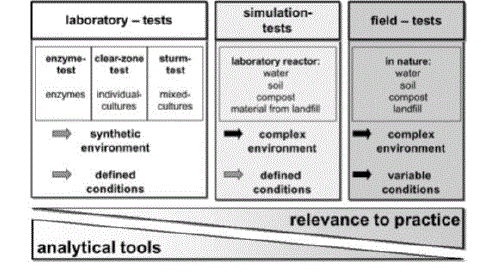
| Test Methods: Tests can be subdivided into three categories: field tests; simulation tests; and laboratory tests (See Figure 9). Various simulation tests in the laboratory have been used to measure the biodegradation of plastics. The degradation might take place in compost, soil or sea-water placed in a controlled reactor in a laboratory. |
| |
| The most reproducible biodegradation tests are the laboratory tests, where defined media are used (in most cases synthetic media) and inoculated with either a mixed microbial population (e.g., from waste water) or individual microbial strains which may have been especially screened for a particular polymer. Such tests may be optimized for the activity of the particular microorganisms used [19]. |
| |
| Factors Affecting Biodegradation of Polymers |
| |
| Factors which may affect the biodegradation of polymers are as follows: |
| |
| • Chemical structure. |
| |
| • Chemical composition. |
| |
| • Distribution of repeat units in multimers. |
| |
| • Presents of ionic groups. |
| |
| • Presence of unexpected units or chain defects. |
| |
| • Configuration structure. |
| |
| • Molecular weight. |
| |
| • Molecular-weight distribution. |
| |
| • Morphology (amorphous/semi crystalline, microstructures, residual stresses). |
| |
| • Presence of low-molecular-weight compounds. |
| |
| • Processing conditions. |
| |
| • Annealing. |
| |
| • Sterilization process. |
| |
| • Storage history. |
| |
| • Shape. |
| |
| • Site of implantation. |
| |
| • Adsorbed and absorbed compounds (water, lipids, ions, etc.). |
| |
| • Physicochemical factors (ion exchange, ionic strength, pH). |
| |
| • Physical factors (shape and size changes, variations of diffusion coefficients, mechanical stresses, stressand solvent-induced cracking, etc.). |
| |
| • Mechanism of hydrolysis (enzymes versus water). |
| |
POLYMERS IN PHARMACEUTICAL APPLICATIONS
|
| |
|
Rosin
|
| |
| Rosin, a film-forming biopolymer, and its derivatives have been extensively evaluated pharmaceutically as film-coating and microencapsulating materials to achieve sustained/controlled drug release. They are also used in cosmetics, chewing gums, and dental varnishes. Rosin is a natural product obtained from the oleoresin of pine trees vizPinussoxburghi and Pinustoeda. |
| |
| Rosin is a low molecular weight (MW = 400) polymer exhibiting excellent film-forming property. It is primarily composed of abietic and pimaric acid, which contain 2 reactive centers: the carboxylic group and the double bonds [20]. |
| |
| Properties |
| |
| 1. Rosin is brittle and friable, with a faint piney odor. |
| |
| 2. It is typically a glassy solid, though some rosin will form crystals, especially when brought into solution. |
| |
| 3. The practical melting point varies with different specimens, some being semi-fluid at the temperature of boiling water, others melting at 100°C to 120°C. |
| |
| 4. It is very flammable, burning with a smoky flame, so care should be taken when melting it. |
| |
| 5. It is soluble in alcohol, ether, benzene and chloroform. Rosin & rosin derivatives are hydrophobic biomaterial which is biodegradable. Good biocompatibility of rosin is demonstrated by the absence of necrosis or abscess formation in the surrounding tissues. [23]. |
| |
|
Gum copal
|
| |
| It occurs in large variety of hard, natural resins produced from a large number of different tree species from many parts of the world - Africa, Asia and South America. Today, most copal of commerce originates from Agathisspecies Family Araucariaceae of Southeast Asia. Copal resin contains agathic acid, a diterpenoid and related lobdane compounds along with cis-communic acid, trans-communic acid. |
| |
| It is a pale yellow transparent crystalline material with softening point range of 79-82 0C. The glass transition temperature is 38.79 0C. Solubility studies of Gum copal revealed that it is hydrophobic in nature. The diffusion of the drug through these films followed zero order kinetics and drug diffusion was extended over a longer period of time at a controlled rate. Hence, these films may be used as rate controlling membranes for the development of transdermal drug delivery systems [21]. |
| |
|
Gum Damar
|
| |
| Gum damar (GD) is a whitish to yellowish natural gum of plant ShoreaWiesneri(family Dipterocarpaceae ). It contains about 40% alpharesin (resin that dissolves in alcohol), 22% beta resin, 23% dammarol acid and 2.5% water. It has been mainly used for water-resistant coating and in pharmaceutical and dental industries for its strong binding properties. Drug release from Gum damar films followed zero order and Higuchi square root kinetics respectively. Hence, due to its substantial matrix forming ability it could be used for sustained drug delivery [22]. |
| |
|
Chitin and Chitosan
|
| |
| Chitin, a naturally abundant mucopolysaccharide, and the supporting material of crustaceans, insects, etc., are well known to consist of 2-acetamido-2- deoxy-b-D-glucose. Chitin can be degraded by chitinase. Chitosan is a linear polysaccharide composed of randomly distributed β-(1-4)-linked Dglucosamine (deacetylated unit) and N-acetyl-Dglucosamine (acetylated unit). It has a number of commercial and possible biomedical uses. |
| |
| Properties |
| |
| Most of the naturally occurring polysaccharides, e.g. cellulose, dextran, pectin, alginic acid, agar, agarose and carragenans, are neutral or acidic in nature; whereas chitin and chitosan are examples of highly basic polysaccharides.Their unique properties include polyoxysalt formation, ability to form films, chelate metal ions and optical structural characteristics. Like cellulose, chitin functions naturally as a structural polysaccharide, but differs from cellulose in its properties. Chitin is highly hydro phobic and is insoluble in water and most organic solvents. It is soluble in hexafluoro- isopropanol, hexafluoroacetone, chloroalcohols in conjugation with aqueous solutions of mineral acids and dimethylacetamide containing 5% lithium chloride. Chitosan, the deacetylated product of chitin, is soluble in dilute acids such as acetic acid, formic acid, etc. Recently, the gel forming ability of chitosan in Nmethylmorpholine N-oxide and its application in controlled drug release formulations has been reported [23]. |
| |
| Chitosan's properties also allow it to be used in transdermal drug delivery. One of these properties is that it is mucoadhesive in nature. Another property that is important to chitosan's ability to aid in drug delivery is that it is fairly reactive so it can be produced in many different forms. The most important property of chitosan with regards to drug delivery is its positive charge under acidic conditions. This positive charge comes from protonation of its free amino groups. Lack of a positive charge means chitosan is insoluble in neutral and basic environments. However, in acidic environments, protonation of the amino groups leads to an increase in solubility. The implications of this are very important to biomedical applications. This is a molecule that will maintain its structure in a neutral environment but will solubilize and degrade in an acidic environment. This means that chitosan can be used to transport a drug to an acidic environment, where the chitosan packaging will then degrade, releasing the drug to the desired environment [24]. |
| |
|
Zein
|
| |
| Zein, an alcohol-soluble protein contained in the endosperm tissue of Zeamais, occurs as a by-product of corn processing. Although zein has been empirically employed as an edible coating for foods and pharmaceuticals for decades, it has not attracted considerable attention as a possible alternative for film-forming agents in drug formulations such as derivatives of cellulose or polyacrylates. Zein might serve as an inexpensive and most effective substitute for the fast-disintegrating synthetic and semisynthetic film coatings currently used for the formulation of substrates that allow extrusion coating [25]. |
| |
|
Pectin
|
| |
| Pectin is a structural hetero polysaccharide contained in the primary cell walls of terrestrial plants.Ther are three pectic polysaccharides, homogalacturonan, rhamnogalacturonan-I and substituted galacturonans. It is produced commercially as a white to light brown powder, mainly extracted from citrus fruits, and is used as a gelling agent, thickening agent and stabilizer.It is also an abundant, ubiquitous and multifunctional component of the cell walls of all land plants. There has been tremendous progress in understanding of the very complex fine structure of pecticpolymers and pectinolytic enzymes. With this increased knowledge comes the prospect of novel applications. The association of pectin chains leads to the formation of various dosage forms wherein it acts as a promising natural polymer for drug delivery. It possesses several requisite characteristics to be used as polymer in drug development and release kinetics. Producers are beginning to develop a new generation of sophisticated designer pectins with specific functionalities [26]. |
| |
|
Collagen
|
| |
| Collagen is the most widely found protein in mammals and is the major provider of strength to tissue. A typical collagen molecule consists of three intertwined protein chains that form a helical structure. These molecules polymerize together to form collagen fibers of varying length, thickness, and interweaving pattern (some collagen molecules will form ropelike structures, while others will form meshes or networks). Collagen may also be processed into a variety of formats, including porous sponges, gels, and sheets, and can be cross linked with chemicals to make it stronger or to alter its degradation rate. The number of biomedical applications in which collagen have been utilized is too high; it not only has been explored for use in various types of surgery, cosmetics, and drug delivery, but in bioprosthetic implants and tissue tissueengineering of multiple organs as well. However, some disadvantages to using collagen as a cell substrate do exist. Depending on how it is processed, collagen can potentially cause alteration of cell behavior, inappropriate mechanical properties, or undergo contraction. Because cells interact so easily with collagen, cells can actually pull and reorganize collagen fibers, causing scaffolds to lose their shape if they are not properly stabilized by cross linking or mixing with another less "vulnerable" material [27]. |
| |
|
Alginates
|
| |
| Alginate is a high molecular weight polymer (a long chain of inter connected atoms). It is a hydrophilic polymer, associated with certain cations (sodium and calcium) which influence its properties and are exchangeable. Sodium alginate is soluble in water; however calcium alginate is insoluble because the calcium ions cross-link polymer chains. A mixed sodium/calcium alginate shows intermediate behavior by absorbing water and swelling to form a gel. |
| |
| The raw material is extracted and purified from seaweed, a natural product. The same plants are harvested on a continuing basis and so are renewable sources. |
| |
| Different polymer types ( Manuronic acid or Guluronic acid) arise from the selection of particular seaweed species and molecular weight is controlled during extraction. The polymer is finally produced as a powder in the soluble, sodium form. Unfortunately, some drawbacks to alginate include mechanical weakness and poor cell adhesion. To overcome these limitations, the strength and cell behavior of alginate have been enhanced by mixtures with other materials, including the natural polymers agarose and chitosan [28]. |
| |
|
Shellac
|
| |
| Shellac is the only known natural resin of animal origin. It is the secretion of a tiny insect known as Kerriarlacca (formally called Laceiferlacca). The lacforming tiny insect grows on some types of trees, mostly abundant in tropical countries, including India. The secretion of the lac insect hardens in air and forms a covering on the body of the insect. This covering or the nest forms a continuous incrustation on the branches of the host trees. Lac is collected by scraping the incrustation from the branches of the trees. |
| |
| It is a natural polymer and is chemically similar to synthetic polymers, and thus can be considered a natural form of plastic. Although advancement in plasticshave rendered shellac obsolete as a moulding compound, it remains popular for a number of other uses. In dental technology, it is still occasionally used in the production of custom impression trays and (partial) denture production [29]. |
| |
|
Xantum gum
|
| |
| This gum is produced by a pure culture fermentation of a carbohydrate with Xanthomonascampestris and purified. It is used as a stabilizer, thickener and emulsifier extensively in pharmaceutical industry. |
| |
|
Starch
|
| |
| Starch is one of the most studied natural polymers for plastic application, owing to its availability, biodegradability, and low cost. Starch exists in a granular form in its natural state, but when subjected to shear forces at a temperature in the range 90-180 °C, in the presence of a plasticizer such as glycerol, starch loses much of its original granular structure and is transformed into a molten plastic state named thermoplastic starch. Unfortunately, thermoplastic starch has two main disadvantages in comparison with most plastics currently in use, namely that it absorbs moisture and exhibits poor mechanical properties. Several approaches have been tried to overcome these drawbacks, such as reinforcement with fibers and inorganic materials and with both degradable and non-degradable polymers. |
| |
|
Starch-Based Polymers
|
| |
| Starch is a natural polymer which possesses many unique properties and some shortcoming simultaneously. Therefore, by combining the individual advantages of starch and other natural polymers, starch-based biodegradable polymers are potential for applications, in the form of microsphere or hydrogel, for drug delivery. |
| |
| Pure starch possesses the characteristic of being able to absorb humidity, and is thus being used for the production of drug capsules in the pharmaceutical sector. Flexibiliser and plasticiser such as sorbitol and glycerine are added so the starch can also be processed thermo-plastically. By varying the amounts of these additives, the characteristic of the material can be tailored to specific needs (also called "thermoplastical starch") [30]. |
| |
|
Silk fibroin
|
| |
| Silk fibroin is well known as biocompatible polymer capable of being easily and largely accessible from nature. It is a natural protein from Bombyxmori, is a semicrystalline polymer mainly composed of glycine, alanine, serine and tyrosine. Recently, it has been studied in fields of bio-industry, such as fibers, hydrogels for controlled drug release, scaffolds for cell culture, films for enzyme immobilized biosensors due to its biocompatible properties and durability in a biological environment. |
| |
|
Carrangeenan
|
| |
| Carrageenan is located in the cell wall and intercellular matrix of the seaweed plant tissue. It is a high molecular weight polysaccharide with 15% to 40% of ester-sulfate content. It is formed by alternate units of D-galactose and 3.6 anhydro-galactose (3.6- AG) joined by α-1,3 and β-1,4 -glycosidic linkage. Hot aqueous solutions of kappa carrageenans have the ability to form thermo-reversible gels upon its cooling. This phenomenon occurs due to the formation of a double helix structure by the carrageenan polymers. |
| |
|
Agarose
|
| |
| Agarose is the main component of the gelatinous agar that can be isolated from certain species of seaweed – is itself a polymer. Chemically, agarose is a polysaccharide, whose monomeric unit is a disaccharide of D-galactose and 3,6-anhydro-Lgalactopyranose. In aqueous solutions below 35°C these polymer strands are held together in a porous gel structure by non-covalent interactions like hydrogen bonds and electrostatic interactions. Heating the solution breaks these non-covalent interactions and separates the strands. Then as the solution cools, these non-covalent interactions are reestablished and the gel forms. |
| |
|
Gelatin
|
| |
| Gelatin is a mixture of peptides and proteins produced by partial hydrolysis of collagen extracted from the boiled bones, connective tissues, organs and some intestines of animals Gelatin is an irreversible hydrolyzed form of collagen, formed by breaking apart its natural triple-helix structure into singlestrand molecules. It is non-immunogenic compared to collagen; |
| |
|
Hyaluronic acid or hyaluronan (HA)
|
| |
| Hyaluronic acid or hyaluronan (HA) is a naturally occurring, water soluble, polysaccharide that is widely distributed throughout the ECM of all connective tissues in human and other animals. It is the only nonsulfated GAG consisting of multiple disaccharide units of glucuronic acid and Nacetylglucosamine. The coiled structure of HA can trap 1000 times its weight in water. These characteristics give it unique physicochemical properties as well as distinctive biological functions. As a consequence, HA and its derivatives have been widely investigated as materials for tissue engineering, for instance, the HA properties include many special interactions with growth factors, receptors and adhesion proteins that suggest that its incorporation into a chitosan-gelatin network it may modify the material bioactivity generating a hydrogel in which other matrix molecules can assemble [31]. |
| |
|
Hydrogels
|
| |
| Hydrogel (also called aquagel) is a network of polymer chains that are hydrophilic, sometimes found as a colloidal gel in which water is the dispersion medium. Hydrogels are highly absorbent (they can contain over 99% water) natural polymers. They also possess a degree of flexibility very similar to natural tissue, due to their significant water content. They are used as reservoirs in topical drug delivery; particularly ionic drugs, as sustainedrelease drug delivery systems. Some of their types are listed below: |
| |
| • Hydrogels from polysaccharide-based materials |
| |
| • Alginate hydrogels |
| |
| • Ethylene-vinyl alcohol hydrogel |
| |
| • Polyelectrolyte hydrogel |
| |
| • Thermo responsive hydrogel poly(N-isopropylacrylamide) |
| |
|
Cellulose-Based Polymers
|
| |
| Cellulose-based plastics are mainly the cellulose esters (cellulose acetate, nitrocellulose etc.) and derivatives of cellulose, have long served for the manufacture of films and fibers. The most useful acetate material is the diacetate, in which two thirds of the cellulose hydroxyl groups have been esterified. Some of the cellulose based polymers are listed below: |
| |
| • Ethyl cellulose Insoluble |
| |
| • Carboxymethyl cellulose |
| |
| • Hydroxyethyl and hydroxypropylcelluloses |
| |
| • Hydroxypropyl methyl cellulose |
| |
| • Cellulose acetate phthalate |
| |
|
Hydrocolloids
|
| |
| A water-swellable hydrocolloid polymer consists essentially of about 80 to about 99.995 mole percent polymerized water-soluble alpha, betamonoethylenically unsaturated monocarboxy monomer containing a three carbon atom chain, zero to about 19.995 mole percent polymerized watersoluble copolymerized monoethylenically unsaturated monomer, and about 0.005 to about 0.10 mole percent polymerized water-soluble copolymerizable cross-linker mixture. |
| |
| • Alginic acid |
| |
| • Carrageenan Modified release,viscosifier |
| |
| • Chitosan controlled drug delivery applications, mucoadhesivedosage forms, rapid release dosage forms |
| |
| • Hyaluronic acid Reduction of scar tissue, cosmetics |
| |
| • Pectinic acid Drug delivery |
| |
|
Water-Insoluble Biodegradable Polymers
|
| |
| • (Lactide-co-glycolide) polymers Microparticle–nanoparticle for protein delivery |
| |
|
Water-soluble Biodegradable Polymers
|
| |
| • Nichigo G-Polymer |
| |
|
Polylactide (PLA)
|
| |
| PLA is actually a polymer of lactic acid, but the dimericlactide is used as the precursor to avoid the water that would be formed in a direct polyesterification. Bacterial fermentation is used to produce lactic acid from corn starch or cane sugar. After dimerization to the lactide, ring-opening polymerization of the purified lactide is effected using stannous compounds as catalysts. PLA can be processed like most thermoplastics into fibers and films. In situations that require a high level of impact strength, the toughness of PLA in its pristine state is often insufficient. Blends of PLA with other polymers have good form-stability and visual transparency, making them useful. PLA materials are currently used in a number of biomedical applications, such as sutures, stents, dialysis media and drug delivery devices. However, one of the drawbacks of polylactides for biomedical applications is their brittleness. |
| |
|
Polyhydroxyalkanoates
|
| |
| PHA (polyhydroxyalkanoates) is synthesized by microorganisms such as Alcaligeneseutrophus, grown in a suitable medium and fed appropriate nutrients so that it multiplies rapidly. Once the population has increased, the nutrient composition is changed, forcing the micro-organism to synthesize PHA. Harvested amounts of PHA from the organism can be as high as 80% of the organism's dry weight. The simplest and most commonly occurring form of PHA is poly (R-3-hydroxybutyrate), PHB or P(3HB)). Pure PHB, consisting of 1000 to 30000 hydroxy acid units, is relatively brittle and stiff. Depending upon the microorganism, many of which are genetically engineered for this purpose, and the cultivation conditions, homo- or copolyesters with different hydroxyalkanic acids may be generated. Such copolymers may have improved physical properties compared with homo P(3HB). Presently, these PHAs cost about twice as much as petroleum-based plastics. An engineered switch-grass that grows PHA inside its leaves and stems has also been created, offering the possibility of avoiding some of the costs associated with large scale bacterial fermentation. |
| |
|
Bio-derived polyethylene
|
| |
| The basic building block (monomer) of polyethylene is ethylene. This is just one small chemical step from ethanol, which can be produced by fermentation of agricultural feedstocks such as sugar cane or corn. Bio-derived polyethylene is chemically and physically identical to traditional polyethylene - it does not biodegrade but can be recycled. It can also considerably reduce greenhouse gas emissions [32]. |
| |
|
Natural rubber
|
| |
| Natural rubber is an addition polymer that is obtained as a milky white fluid known as latex from a tropical rubber tree. Natural rubber is from the monomer isoprene (2-methyl-1,3-butadiene). |
| |
| Various hydrocolloids or polysaccharide gums are originated from a variety of sources. Most gums are hydrophilic and contain very long polymeric chains as well as different functional groups. These features are very attractive in many pharmaceutical processes such as coating, stabilization, thickening, binding, solubilization, and disintegration. Gums behave differently in water and aqueous solutions. Almost all display thickening property, whereas some show gelling property. Although thickening is a desirable property for solution, suspension, and emulsion dosage forms, gelling property is utilized in drug encapsulation for controlled delivery applications. |
| |
| Gums such as guar gum can provide excellent thickening property. Guar gum a polysaccharide derivative with glycoside linkage has been used as matrix former for controlled release of isoniazide and diltiazem. Gum Arabic or gum acacia is best known for its emulsifying property and its solution viscosity at very high solid concentration. Locust bean gum consists of mannose and galactose sugar units at a ratio of 4:1. Like almost all gum solutions, an aqueous solution of this gum displays shear thinning rheology. It shows synergistic effect with xanthan and kappa carrageenan. Gellan gum has been used in pharmaceutical dosage forms as a swelling agent, as a tablet binder, and as a rheology modifier. Generally speaking, as opposed to branched gums, linear gums have more sites available for intermolecular hydrogen bonding; as a result, they offer better filmforming properties. |
| |
| Individual and blended gum products based on agar, alginate, κ-carrageenan, methyl cellulose, pectin, CMC, and guar can potentially be used in film dosage forms. Gums can easily be derivatized to change their solution properties. Xanthan gum is found in a number of drug formulations including cefdinir oral suspension and nitazoxanide tablets. It is a highly branched glucomannan polysaccharide with excellent stability under acidic conditions. Xanthan is generally used in solution and suspension products for its thickening property. Because of its very rigid structure, its aqueous solution is significantly stable over a wide pH range. |
| |
| Alginic acid and its salts are anionic polymers that can offer gelling properties. They have found applications as a stabilizing agent, binding agent, drug carrier, and so on. The antibiotic griseofulvin, which is supplied as oral suspension, contains sodium alginate stabilized with methylparaben. Alginic acid and ammonium calcium alginate can be found in metaxalone tablets. Alginate microbeads can be used to entrap drugs, macromolecules, and biological cells [33]. |
| |
|
Environmental Impacts of Biopolymers
|
| |
| Engineers are attempting to integrate environmental considerations directly into material selection processes, in order to respond to an increased awareness of the need to protect the environment. The use of renewable resources in the production of polymer materials achieves this in two ways. First of all, the feed stocks being employed can be replaced, either through natural cycles or through intentional intervention by humans. The second environmental advantage of using renewable feed stocks for biopolymer development is the biodegradable nature of the end products, thereby preventing potential pollution from the disposal of the equivalent volume of conventional plastics. At the end of their useful period, biopolymer materials are generally sent to landfills or composted [34]. |
| |
|
Future trends
|
| |
| Despite the excessive use of synthetic polymers the need for natural biodegradable polymers to deliver drugs continues to be area of active research. Natural polymers have numerous advantages over synthetic ones as being readily available, relatively inexpensive, natural products of living organisms, possibilities of chemical modifications [35]. |
| |
| The most exciting opportunities in polymer drug delivery lie in the arena of responsive delivery systems, with which it will be possible to deliver in response to a measured blood level or to deliver a drug precisely to a targeted site. Much of the development of novel materials in controlled drug delivery is focusing on the preparation and use of these responsive polymers with specifically designed macroscopic and microscopic structural and chemical features [36]. |
| |
| Such systems include: |
| |
| • Copolymers with desirable hydrophilic/hydrophobic interactions. |
| |
| • Block or graft copolymers. |
| |
| • Complexation networks responding via hydrogen or ionic bonding. |
| |
| • Dendrimers or star polymers as nanoparticles for immobilization of enzymes, drugs, peptides, or other biological agents. |
| |
| • New biodegradable polymers. |
| |
| • New blends of hydrocolloids and carbohydrate-based polymers. |
| |
| These new biomaterials tailor-made copolymers with desirable functional groups are being created by researchers who envision their use not only for innovative drug delivery systems but also as potential linings for artificial organs, as substrates for cell growth or chemical reactors, as agents in drug targeting and immunology testing, as biomedical adhesives and bio separation membranes, and as substances able to mimic biological systems. Novel supramolecular structures based on polyethylene oxide copolymers and dendrimers are being intensively researched for delivery of genes and macromolecules [37]. |
| |
| Design and synthesis of novel combinations of polymers will expand the scope of new drug delivery systems in the future. This will obviously require assimilation of a great deal of emerging information about the chemical nature and physical structure of these new materials. |
| |
| There is an increasing movement of scientists and engineers who are dedicated to minimizing the environmental impact of polymer composite production. Life cycle assessment is of paramount importance at every stage of a product's life, from initial synthesis through to final disposal and a sustainable society needs environmentally safe materials and processing methods[38]. |
| |
Conclusion
|
| |
| Biodegradable polymers have proven their potential for the development of new, advanced and efficient drug delivery systems. They are capable of delivering a wide range of bioactive materials. Latest developments in the active transdermal drug delivery system to use novel techniques like X-ray lithography &LIGA (Lithography, Electroplating, and Molding), mechanical array, electro oration, ultrasound, etc. indicate that efforts are underway to increase the range of drugs that can be delivered transdermally. With the wide range of polymers that are being used in the transdermal drug delivery systems, there are still issues faced in terms of skin side effects like rash, inflammation, burn; crystallization of the drug in the rate-controlling membrane altering the rate of permeation of drug in the skin, etc. All these disadvantages suggest that more research and testing of materials being employed is required before they are put out for commercial use. |
| |
Acknowledgment
|
| |
| The authors gratefully acknowledge the support provided by the KIET School of pharmacy, Ghaziabad for providing necessary guidance and facilities at the time of work. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
 |
 |
 |
 |
| Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
|
| |















