Keywords
Aphthous; Recurrent Aphtous Stomatitis (RAS); Treatment; Herbal; Natural
Introduction
Recurrent Aphthous Stomatitis (RAS) is one of the most common pathologic conditions with ulcers in the oral mucosa. RAS is manifested in the oral mucosa, as a single or multiple recurring ulcer, painful with erythematous halo [1,2]. Epidemiologic studies have reported an average prevalence of 17% of the total population [2,3]. A successful treatment of aphthous stomatitis requires proper diagnosis and control of possible etiologic factors. None of the treatments have been satisfactory due to the widespread factors; however, there is a wide range of them. The etiology of aphthous stomatitis is not fully known [4-10]. Treating aphthous stomatitis is symptomatic and is mainly based on empirical evidence [5]. Prescribed drugs must be related to the severity of the disease [2]. Conventional aphthous stomatitis treatments include antiseptics, anti-inflammation, analgesics, antibiotics, corticosteroids, local anesthesia, lasers and herbal medicine [11]. Therefore, corticosteroids and analgesics are the first choices for RAS patients [12]. Yet, longer treatment and frequent intake of these drugs might cause severe complications such as secondary fungal infections and drug resistance. In most cases, the aim of RAS treatment is believed to reduce the pain, disease duration, and frequency of relapses [13,14]. RAS is a chronic periodic oral mucosal disorder that can adversely affect the everyday life, such as physical health, pain and oral function [4]. Evidence shows that the chronic conditions of mucus have a major impact not only on physical functioning but also on the psychological and social aspects [15]. Due to RAS high prevalence, prevention, pain reduction, or reduced disease duration are the most important goals in dentistry [4]. Although herbal medicines are widely used in Iran and other countries and multiple studies are conducted in this regard, a single treatment or even a preferred choice has not been introduced for RAS. Therefore, a review study is needed to select the appropriate drug for prescribing in clinics. This research aimed to investigate and analyze the efficient of herbal drugs used for treating RAS. A systematic review was conducted.
Materials and Methods
This descriptive and analytical study has been carried out in a systematic review form in Mashhad Faculty of Dentistry, Iran, 2016. The aim of this study is critique of the clinical trial methodology published in Persian and English journals in terms of natural and herbal treatments or RAS prevention from 2010 to 2015.
Since frequent studies were conducted in this regard in this period, we aimed at using the latest and best articles to select the optimal treatment. This is a systematic review and the outcome was not measurable. The overall score of the articles were measured by the Cochrane Collaboration’s tool for assessing risk of Bias.
All clinical trials with topical and systemic therapeutic interventions aimed at the treatment or prevention of relapses printed in English and Persian, related to the effect of natural and herbal medicines on RAS treatment or prevention were searched from the beginning of 2010 to the end of 2015 using the following keywords based on PICO at Pubmed, ISI web of science, Scopus, Cochrane, OVID:
P: Patients diagnosed with primary aphthous ulcers
I: Natural and herbal medicines
C: Not used
O: RAS prevention or treatment
C and O are not very important when the search is based on the P and I. Aphthous stomatitis and oral ulcers were separately searched in Persian databases (SID and Iranmedex).
Inclusion criteria
1. Clinical trials published in relation to the effective herbal or natural drugs in treating or preventing RAS.
2. Persian or English articles.
3. Local and systematic interventions to prevent the relapse.
4. Studies from the beginning of 2010 to the end of 2015.
Exclusion criteria
1. Studies related to Aphthous-like ulcers.
2. Studies in which related syndromes were taken into account such as Behcet, Reiter's syndrome, or other pseudoportal ulcers, Crohn's disease, ulcerative colitis and anemia, or drug use.
Data separation and quality assessment of studies
Two researchers investigated the topic and abstract in terms of the inclusion criteria. The selected articles were used for the next stage. The researchers agreed on the conflicts after negotiation. Then, the selected articles were investigated in terms of scientific principles, inclusion criteria, and methodology. The references were manually checked in order to consider related articles in case the inclusion criteria were met. To extract the data, a structured, standard form was used. Topic, Journal, Year of Publishing, Country, and Corresponding's Course of Study were recorded. To search for the bias, Risk of Bias, used in systematic reviews developed by the Cochrane Group (last update in 2011), was employed [16]. This reliable, valid tool is used in all randomized clinical trial regardless of language, time, and location of publishing. The tool consists of six dimensions: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias. The articles were investigated by two referees. In case of disagreement, the third referee was hired. The final comment was afterward given on the article. Each of the articles was reported in three ways: low risk, high risk, and vague risk. Finally, the overall scores of the articles were determined according to the Cochrane Collaboration’s Tool for assessing risk of bias. "Yes" was summed up for every article. If the total score was greater than 5, the article was considered low risk. If the totals core was between 3 and 5, it was considered medium risk. Scores over 3 were considered high risk.
Data analysis
Since the risk of bias was not low, statistical analysis (metaanalysis) was not carried out.
Results
A total of 7485 articles were selected using the electronic search. 5949 articles remained in the study after eliminating the repetitive results. 5902 studies were removed by investigating the topic. The abstracts of 47 studies were investigated. Out of 47, the full text of 5 articles was not found. Two articles had abstracts. Two were in-vitro; two were systematic; two were carried out in 2005; and one study was conducted on animals. A total of 13 out of 48 articles were eliminated. Finally, a sample of 33 articles was selected (Figure 1). Treatments varied in the trails.
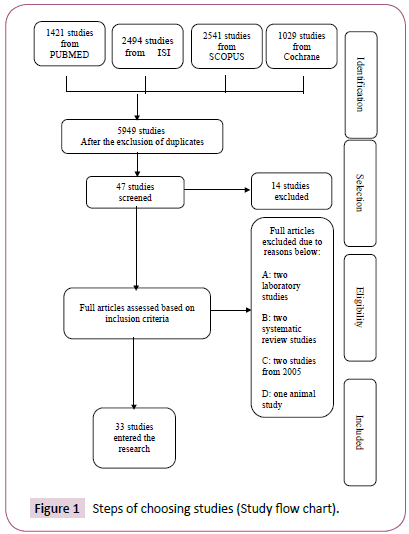
Figure 1: Steps of choosing studies (Study flow chart).
Random assignment (unpredictable random sequence)
Out of 33 articles, 12 had random assignment of which two had medium risk [17,18]; 10 had high risk [19-33]; one study had no random assignment and had high risk [21]; and 20 had unclear random assignment of which 4 had medium risk [34-36] and 16 had high risk [5-8,17,18,22,26,37-45]. Out of 33 articles, 32 had unclear random allocation concealment (Tables 1 and 2) and one article with medium risk [28] had clear random allocation concealment.
| Lead author |
Year |
Questions |
Bias amount |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
| Aswath |
2014 |
? |
? |
- |
- |
? |
- |
- |
Strong bias |
| Bhalang |
2013 |
? |
? |
? |
- |
? |
+ |
- |
Strong bias |
| Najafi |
2013 |
+ |
? |
+ |
+ |
+ |
+ |
- |
Strong bias |
| Rad |
2010 |
+ |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Eslami Raveshty |
2011 |
- |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Katti |
2011 |
? |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Mansour |
2013 |
+ |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Jiang |
2012 |
+ |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Raeesi |
2015 |
+ |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Manifar |
2012 |
? |
? |
? |
- |
+ |
- |
- |
Strong bias |
| Pourahmad |
2010 |
? |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Guintu |
2013 |
+ |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Jiang |
2013 |
+ |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Hoseinpour |
2011 |
+ |
? |
+ |
- |
+ |
- |
- |
Strong bias |
| Khademi |
2014 |
? |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Babaee |
2012 |
? |
? |
+ |
- |
- |
+ |
- |
Strong bias |
| El-Haded |
2014 |
+ |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Hamdy |
2010 |
? |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Romero-Cerecero |
2015 |
? |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Liu |
2012 |
? |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Haghpana |
2015 |
? |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Halim |
2013 |
? |
? |
- |
- |
+ |
+ |
- |
Strong bias |
| Bechir |
2014 |
? |
? |
- |
- |
? |
- |
- |
Strong bias |
| Gavanji |
2013 |
? |
? |
? |
- |
+ |
+ |
- |
Strong bias |
| Ali |
2011 |
? |
? |
? |
- |
+ |
+ |
- |
Strong bias |
| Gavanji |
2014 |
? |
? |
+ |
- |
+ |
+ |
- |
Strong bias |
| Babaee |
2010 |
? |
? |
+ |
+ |
- |
- |
- |
Strong bias |
| He |
2014 |
+ |
? |
? |
- |
+ |
- |
- |
Strong bias |
| Ghalayani |
2013 |
? |
? |
+ |
- |
? |
+ |
- |
Medium bias |
| Stojanovska |
2015 |
? |
? |
+ |
- |
? |
+ |
- |
Medium bias |
| Seyyedi |
2014 |
+ |
? |
+ |
+ |
- |
+ |
- |
Medium bias |
| Sukumaran |
2010 |
? |
? |
? |
- |
+ |
+ |
- |
Medium bias |
| Deshmukh |
2014 |
+ |
- |
? |
- |
+ |
+ |
- |
Medium bias |
Table 1: Bias assessment items.
| Unknown (unclear) number (Percentage) |
No (Strong bias) Number (Percentage) |
Yes (Low bias) number (Percentage) |
Questions |
| 20 (60.6) |
1 (3.0) |
12 (36.4) |
1.Was the random assignment procedure correct? |
| 32 (97.0) |
10 (3.0) |
0 (0) |
2. Was the allocation concealment enough? |
| 7 (21.3) |
9 (27.3) |
17 (51.5) |
3. Was the blinding carried out properly? (participants, medical staff) |
| 0 (0) |
30 (90.9) |
3 (9.1) |
4. Was the statistical analyst blinding carried out? |
| 5 (15.2) |
3 (9.1) |
25 (75.8) |
5. Are the incomplete data results reported? |
| 0 (0) |
6 (18.2) |
27 (81.8) |
6. Did the study assess and report the intended outcome? |
| 0 (0) |
33 (100) |
0 (0) |
7. Is the study clear of other bias risks? |
Table 2: Cocharane check list answer frequency.
Blinding participants, medical staff, and statistical analyst
Out of 34 articles, blinding for participants and medical staff was carried out in 17 studies. Out of 17 articles, only 3 had medium risk [29,35,36] and 14 had high risk. Out of 34 articles, blinding was carried out in 3 articles for the statistical analyst of which two had high risk [5,19] and one had medium risk [29] (Tables 1 and 2).
Incomplete outcome data reporting
Out of 33 articles, 3 had incomplete data of which two had high risk [5,38] and one had medium risk [29]. No incomplete data were found in 25 articles of which two had medium risk [28,34] (Tables 1 and 2).
Selective reporting
Out of 33 articles, no selective reporting was found in 28 articles of which 5 had medium risk [28,29,34-36].
Other bias
All 33 articles had at least one bias including the study plan or misconceptions, early termination of the study, heterogeneity in basic information, and other problems in the study, of which 5 studies had a medium bias.
The lowest bias was related to the selective reporting (81.7%) followed by missing data (75.8%). The greatest bias was related to other bias (100%) and statistical analyst blinding (90.9%). Table 3 shows the variables used in the study (Figure 2) (Table 2).
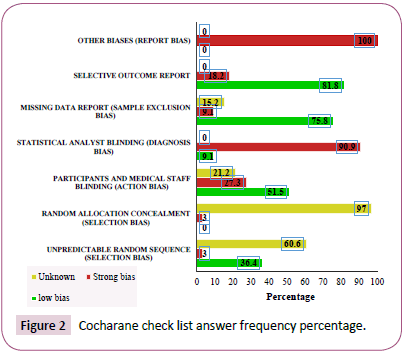
Figure 2: Cocharane check list answer frequency percentage.
| Lead author |
Year |
Lead author’s address |
Country |
Journal |
| Aswath N |
2014 |
Department of Oral Medicine and Radiology |
India |
RJPBCS |
| Bhalang K |
2013 |
Faculty of Dentistry |
Thailand |
J Altern Complement Med |
| Najafi Sh |
2013 |
Oral disease |
Iran |
TUMJ |
| Rad F |
2010 |
Dermatologist |
Iran |
Armaghan danesh |
| Eslami, Raveshty SS |
2011 |
Director of Research and Development of the Pharmaceutical Company |
Iran |
ZUMS Journal |
| Katti G |
2011 |
Department of Oral Medicine and Radiology |
India |
Int. j. dent. clin. |
| Mansour Gh |
2013 |
Department of Oral Basic and Clinical Sciences |
Saudi Arabia |
J Oral Pathol Med |
| Jiang XW |
2012 |
Department of Stomatology |
China |
Oral Surg Oral Med Oral Pathol Oral Radiol |
| Raeesi VR |
2015 |
Department of Internal Medicine |
Iran |
Acta Medica Mediterranea |
| Manifar S |
2012 |
Oral Medicine Clinic |
Iran |
Journal of Medicinal Plants |
| Pourahmad M |
2010 |
Department of Internal Medicine |
Iran |
JDDG |
| Guintu FZ |
2013 |
Department of Otorhinolaryngology |
Philippines |
Philipp J Otolaryngol Head Neck Surg |
| Head and Neck Surgery |
| Jiang XW |
2013 |
Department of Stomatology |
China |
Oral Surg Oral Med Oral Pathol Oral Radiol |
| Hoseinpour H |
2011 |
Department of Oral Medicine |
Iran |
Quintessence Int |
| Khademi |
2014 |
Dental Material Research Centre and Department of Oral Medicine |
Iran |
Int Sch Res Notices |
| Babaee N |
2012 |
Department of Oral Medicine and Diagnosis |
Iran |
Dent Res J (Isfahan) |
| El-Haded SA |
2014 |
Department of Periodontology |
Saudi Arabia |
Quintessence Int |
| Hamdy AA |
2010 |
Department of Oral Medicine and Periodontology |
Egypt |
J Contemp Dent Pract |
| Romero-Cerecero O |
2015 |
Medical Research Center |
Mexico |
J Ethnopharmacol |
| Liu X |
2012 |
Department of Oral Medicine |
China |
Evid Based Complement Alternat Med |
| Haghpana P |
2015 |
Department of Periodontology |
Iran |
Caspian J Intern Med |
| Halim, DS |
2013 |
School of Dental Sciences |
Malaysia |
International Medical Journal |
| Bechir A |
2014 |
Department of Dental Specialties |
Romania |
Revista de Chimie |
| Gavanji S |
2013 |
Department of Biotechnology, Faculty of Advanced Sciences and Technologies |
Iran |
IJSRIN |
| Ali HS |
2011 |
Department of Pharmaceutics and Pharmacy Practice |
Dubai |
Asian J Pharm Clin Res |
| Gavanji Sh |
2014 |
Young Researchers and Elite Club |
Iran |
integr med res |
| Babaee N |
2010 |
Department of Oral Medicine |
Iran |
Clin Oral Investig |
| He Y |
2014 |
School of Stomatology |
China |
J Kuwait Med Assoc |
| Ghalayani P |
2013 |
Department of Oral Medicine |
Iran |
J Res Pharm Pract |
| and Torabinejad Dental Research |
| Stojanovska AA |
2015 |
Department of Oral Pathology and Periodontology |
Macedonia |
PRILOZI |
| Seyyedi SA |
2014 |
Assistant Professor. Faculty of Dentistry |
Iran |
J Clin Exp Dent |
| Sukumaran VG |
2010 |
Sree Balaji Dental College and Hospital |
India |
P R O B E |
| Deshmukh RA |
2014 |
Department of Oral Medicine and Radiology |
India |
Int J Pharm Investig |
Table 3: Variables used in the research studies.
Interventions
1. Tablets containing synbiotic lozenges [17]
2. Acemannan polysaccharide aloe vera extract [18]
3. Purslane extract [19]
4. Myrtle solution [20]
5. Dough containing Myrtus communis (Myrtle) [5]
6. Combination of Dracocephalum and Myrtle essence [21]
7. Amlexanox [22]
8. Adhesive oral tablets (allicin (diallyl thiosulfinate) [24]
9. Licorice Glycyrrhiza glabra biological paste [25]
10. Curcumin gel (contains turmeric) [8,28]
11. Camel Thorn [26]
12. Guava mouthwash leaves (Psidium guajava) [27]
13. Berberne gelatine [33]
14. Rosa damascena mouthwash [32]
15. Iralvex essence [43]
16. Aloe vera [23,38]
17. Honey [30,41]
18. Quersetin [42]
19. Ageratina pichinchensis essence [45]
20. Yunnan Baiyao [44]
21. Ginger officinale extract [7]
22. Combination of collagenic gel and marine algae extract [39]
23. New herbal medicine formulation [40]
24. Propolis buccal paste [37]
25. Punica granatum [6,35]
26. Kasmitad gel [31]27. Proaftol spray [36]
28. Chamomilla tincture mouthwash [29]
29. Polyherbal formulation (HiOra-SG gel) [34]
Discussion
There is a long history of using natural herbal medicines for various diseases, including RAS, around the world. Such treatments have been evaluated in both clinical and experimental studies [6,7,12,23,24,35,41]. This article aimed to find the best natural and herbal treatment for oral aphthous ulcers. 33 clinical trials were selected. The effectiveness of 29 herbal medicines was evaluated for RAS treatment. Out of 33 articles, 28 had high risk and 5 had medium risk [28,29,34-36]. A significant heterogeneity was found due to the comparisons, the type and timing of the evaluation of the results. Similar methods were not used in these studies. Some studies reported the effects of herbal medicines in 5, 7-day periods.
The criteria and evaluation timing varied in every research. None of the interventions reported clear effect on RAS. Homogeneity of studies was very poor in evaluating variables such as type of treatment, dose, formula, method, sample size, and duration of testing, positive and negative control group, follow up time, inclusion and exclusion criteria. Meanwhile, similar methods were not used for evaluation. Each study had used and evaluated only one kind of herbal medicine, therefore a lack of singular evaluation method made comparing the medicines effects, and finding the most effective medicine impossible. The effectiveness of the findings is unclear for the patients diagnosed with RAS due to poor reporting, high risk, and an average count of examined interventions. As a result, meta-analysis was not performed. This article shows the need for the well-designed clinical trials. Such clinical trials are of great importance for the future studies. It is also necessary to develop accurate and standard methods to ensure the quality of the data. In this review study, sequence generation was based on the random numbered table, random sequence generation software, coin tossing, card, draw, minimization, etc. in 12 studies (36.4%). In 20 studies (60.6%), insufficient information for a positive or negative judgment in terms of the random sequence generation made these studies unclear. In terms of the random allocation concealment, insufficient information was found in 32 articles (97.0%) for the positive or negative judgment. In 10 studies (3.0%), the participants or the researcher was able to guess the allocation of groups such as the use of clear, transparent letters, every-other allocation, or allocation based in birth date, etc. In a general definition, bias is the absence of impartiality and deviation from the truth. Bias can happen in all stages of a study from the design to publishing. The best method to reduce the bias is randomization. Randomization is of great importance so that the results of a study showed that incorrect sequence generation and allocation overestimated the effect by 30% to 50% compared to the one using the proper method. In fact, low-quality studies tend to exaggerate the results [46]. The results of this article indicated that in 17 (51.5%) studies, for key positions participants and health professionals were either blinded or not, and those who were not blinded did not cause a bias risk. In 9 studies (27.3%), the results were likely to be affected by lack of blinding or blinding, carried out for key participants, was leaked and failed. 7 studies (21.3%) had unclear situation in this regard. In terms of blinding the statistical analyst, blinding was used only in three studies (9.1%) and blinding was not carried out in 30 studies (90.9%). Blinding can help reduce the bias so that it promotes the achievement of real results and adverse effects caused by the knowledge of researchers and participants on the results. Blinding assessor can help prevent the measurement bias to some extent. Blinding usually reduces the different analysis of the results (information bias) and improves the acceptance of participants and their survival by reducing the bias caused by awareness effects. The results of a study showed that lack of blinding in clinical trials increased the effect of estimation by 90% [46].
In 25 studies (75.8%), either no missing data were found, or the missing data were not related to the main variables or the missing data had the same count and reason in the control and intervention groups. In 3 studies (9.1%), the data reposting was incompletely associated with the correct results. In intervention and control groups, the number and reason of missing data are not similar. The missing data are quite large, which could have affected the results. In 5 studies (15.2%), lack of sufficient information was found in terms of the number and reasons of excluding the participants for the positive or negative judgment. Missing data which are cause by sample drop or exclusion from study, are responsible for bias. The elimination of the results caused by missing data at the analysis stage leads to the results in favor of the control group. Therefore, all RCTs must point the reason of leaving the study. They also need to report the Intention to Treat analysis [46]. In 27 studies (81.8%), the proposal is available, and all predetermined consequences were reported. At the same time, the proposal was not available; however, the results clearly covered the expected results. In 6 studies (18.2%), either all initial consequences were not reported, or some reported consequences used tools not mentioned in the proposal. Few consequences were incompletely reported. In some cases, expected key consequences were not reported.
In all studies (100%), there was at least one significant bias including the incorrect tool or proposal, early termination of the study, heterogeneity in basic information, and other problems. The results of this review study showed that the lowest bias was related to the selective reporting (81.7%) followed by missing data (75.8%). The greatest bias was related to other bias (100%) and statistical analyst blinding (90.9%). Similarly, the review study by Li showed that the least bias was related to the selective reporting and missing data report by over 60%. The greatest bias was associated with personnel, participant, and statistical assessor blinding by over 30% [12].
In this review study, certain studies were excluded including those related to Aphthous-like ulcers or studies with focus on Behcet, Reiter's syndrome, etc. Result measurement and indicators must be clearly stated in future studies. According to the current data, the ulcer size, the duration of the lesion and pain severity are usually considered the main indicators. Researchers have not used a standard method for evaluating these indicators. The herbal medicines are diverse. Researchers have not prescribed similar dosages for a single disease. The effects of herbal medicines used in RAU treatment include anti-inflammatory, analgesic, topical anesthetic, antifungal, antibacterial, antiviral, and immunomodulatory effects [47].
The review study by Tarakji on RAS diagnosis and treatment guidelines for dentists aimed at highlighting the main points that GPs should consider. They concluded that there is still no clear and decisive treatment for RAS due to the variety of underlying factors. The treatment aims to reduce pain, number, and size of lesions and increase the disease-free periods [47-49]. The treatment method should be based on the pain severity, the medical history, the frequency of relapse, and the patient's ability to tolerate. Some patients have developed RAS for few-day periods and only a few relapses in a year. Such patients require relief for pain and must maintain their oral health. Drug therapy is considered for patients who experience multiple RASs, that is, every month or have symptoms of severe pain and difficulty in eating. GPs should determine possible nutritional deficiencies or allergies that trigger the onset of an illness before they start using drugs for RAS.
Similar to our study, the review study by Li on the effectiveness and safety of topical treatment using Herbal Medicine in RAS showed that size and duration of lesion and removal of pain were considered the main outcome variables. Cochrane List was used to evaluate the validity. 13 studies were selected. Meta-analysis was not carried out due to the heterogeneity of studies. Age, gender, and race were not taken into account as inclusion criteria. The results showed that topical treatment with herbal medicine appears to be in favor of RAS patients due to reduced ulcer size, shortened ulcer duration, and pain relief without severe complications. As a result, evidence is found on the effectiveness of the herbal medicine due to the RAS final criteria improvement and fewer complications [12]. In this study, we were not able to achieve a single outcome due to the large volume of studies, responsible for diverse methods and consequences. Both studies, however, show that the greatest bias was associated with blinding. It is recommended to determine the most common errors and avoid using them in designs. Proper guidelines are advised to design every clinical trial and avoid the errors. It is also essential to determine a constant indicator in order to investigate the effect of multiple drugs on aphthous to enable the comparison of results.
Cochrane risk of Bias tool (modified) for quality assessment of randomized controlled trials (Tables 4 and 5).
| Study Validity Domains |
Assessment* |
| 1. Sequence generation: Was the allocation sequence adequately generated? |
• Yes |
| š• No |
| • Unclear |
| 2. Allocation Concealment: Was the sequence generation adequately concealed before group assignments? |
• Yes |
| • No |
| • Unclear |
| 3. Blinding of participants and personnel: Was knowledge of the allocated interventions adequately hidden from the participants and personnel after participants were assigned to respective groups? |
• Yes |
| • No |
| • Unclear |
| 4. Blinding of outcome assessors: Was knowledge of the allocated interventions adequately hidden from the outcome assessors after participants were assigned to respective groups? |
• Yes |
| • No |
| • Unclear |
| 5. Incomplete outcome data: Were incomplete outcome data adequately addressed? |
• Yes |
| • No |
| • Unclear |
| 6. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting? |
• Yes |
| • No |
| • Unclear |
| 7. Other sources of bias: Was the study apparently free of other problems that could put it at a risk of bias? |
• Yes |
| • No |
| • Unclear |
Study Quality†:
Table 4: Cochrane risk of bias tool (modified) for quality assessment of randomized controlled trial.
| Cochrane Risk of Bias Tool [Modified] – Judging Criteria |
1. Sequence generation
Yes: If sequence generated by referring to a random number table; Using a computer random number generator; Coin tossing; Shuffling cards or envelopes; Throwing dice; Drawing of lots.
No: If sequence generated by odd or even date of birth; by some rule based on date [or day] of admission or hospital or clinic record number. Allocation by judgment of the clinician; by preference of the participant; by availability of the intervention or based on the results of a laboratory test.
Unclear: Insufficient information to permit judgment. E.g. Stating that “Randomization was done” without providing the details of what was done. |
2. Allocation Concealment
Yes: Participants and investigators enrolling participants could not foresee assignments before assigning subjects to groups because of the use of any of the following: Use of central allocation [including telephone, web-based, and pharmacy-controlled, randomization]; Sequentially numbered drug containers of identical appearance; Sequentially numbered, opaque, sealed envelopes.
No: If participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: Using an open random allocation schedule [e.g. a list of random numbers]; Assignment envelopes used without appropriate safeguards [e.g. use of unsealed, non-opaque or not sequentially numbered envelopes]; Alternation or rotation; Date of birth; Case record number; Any other explicitly unconcealed procedure.
Unclear: Insufficient information to permit judgment. E.g. Use of assignment envelopes is described, but it remains unclear if they were sequentially numbered, opaque and sealed. |
3. Blinding of participants and personnel
Yes: Blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken; No blinding or incomplete blinding, but in the reviewer’s judgment the outcome is not likely to be influenced by lack of blinding.
No: Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding; No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding;
Unclear: Insufficient information to permit judgment. |
4. Blinding of outcome assessors
Yes: Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; No blinding of outcome assessment, but in the reviewer’s judgment the outcome measurement is not likely to be influenced by lack of blinding.
No: Blinding of outcome assessment attempted, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding; No blinding of outcome assessment and the outcome measurement is likely to be influenced by lack of blinding.
Unclear: Insufficient information to permit judgment. |
5. Incomplete outcome data:
Yes: No missing outcome data or loss to follow-up <10%; Reasons for missing outcome data mentioned and are unlikely to be related to true outcome; Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; Use of ‘Intention-to-treat’ analysis; Missing data have been imputed using appropriate methods.
No: Loss to follow-up >10%; Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; ‘As-treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; Potentially inappropriate application of simple imputation.
Unclear: Insufficient reporting of attrition/exclusions to permit judgment |
6. Selective outcome reporting:
Yes: The study protocol is available, and all the study’s pre-specified outcomes of interest have been reported in the pre-specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified.
No: Not all of the study’s pre-specified primary outcomes have been reported; One or more primary outcomes are reported using measurements, analysis methods or subsets of the data [e.g. subscales] that were not pre-specified; One or more reported primary outcomes were not pre-specified [unless clear justification for their reporting is provided, such as an unexpected adverse effect]; One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta-analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear: Insufficient information to permit judgment. |
7. Other sources of bias:
Yes: The study appears to be free of other sources of bias.
No: There is at least one important risk of bias. E.g. the study had a potential source of bias related to the specific study design used; or has been claimed to have been fraudulent; or had some other problem.
Unclear: Insufficient reporting of attrition/exclusions to permit judgment |
Table 5: Cochrane risk of bias tool (Modified)– judging criteria.
Conclusion
conclusion, the current data showed that natural RAS topical treatments have desirable effects and no study has reported any side effects. However, we were not able to select the best herbal medicine due to very poor reports and heterogeneous studies.
21221
References
- Müller S, Pan Y, Li R, Chi AC (2008) Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971-2006. The Emory University experience. Head Neck Pathol 2: 60-66.
- Burket LW, Greenberg MS, Glick M (2003) Burket's oral medicine: Diagnosis and treatment. PMPH-USA.
- Ship JA (1996) Recurrent Aphthous stomatitis. An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 141-147.
- Taylor J, Brocklehurst P, Glenny AM, Walsh T, Tickle M, et al. (2013) Topical Interventions for RecurrentAphthous stomatitis(mouth ulcers). The Cochrane Library, USA.
- Babaee N, Mansourian A, Momen-Heravi F, Moghadamnia A, Momen-Beitollahi J (2010) The Efficacy of a paste containing Myrtus communis (Myrtle) In the management of recurrent Aphthous stomatitis: A randomized controlled trial. Clin Oral Investig 14: 65-70.
- Gavanji S, Larki B, Bakhtari A (2014) The effect of extract of Punica granatum var. pleniflora for treatment of minor recurrent Aphthous stomatitis. Integr Med Res 3: 83-90.
- Haghpanah P, Moghadamnia AA, Zarghami A, Motallebnejad M (2015) Muco-bioadhesive containing ginger officinal e extract in the management of recurrent Aphthous stomatitis: A randomized clinical study. Caspian J Intern Med 6: 3-8.
- Manifar S, Obwaller A, Gharehgozloo A, Boorboor Shirazi Kordi H, Akhondzadeh S (2012) Curcumin gel in the treatment of minor aphthous ulcer: A randomized, placebo- controlled trial. J Med Plant 1: 40-45.
- Parolia A, Thomas MS, Kundabala M, Mohan M (2010) Propolis and its potential uses in oral health. Int J Med Med Sci 2: 210-215.
- Samet N, Laurent C, Susarla SM, Samet-Rubinsteen N (2007) The effect of Bee propolis on recurrent Aphthous stomatitis: A pilot study. Clin Oral Investig 11: 143-147.
- Belenguer-Guallar I, Jimenez-Soriano Y, Claramunt-Lozano A (2014) Treatment of recurrent aphthous stomatitis. A literature review. J Clin Exp Dent 6: e168-174.
- Li CL, Huang HL, Wang WC, Hua H (2016) Efficacy and safety of topical herbal medicine treatment on recurrent Aphthous stomatitis: A systemic review. Drug Des Devel Ther 10: 107-115.
- Pankhurst C (2006) Practical oral medicine. Primary Dental Care 13: 96.
- Scully C, Shotts R (2000) ABC of oral health. Mouth ulcers and other causes of orofacial soreness and pain. BMJ 321: 162-165.
- Ni Riordain R, Meaney S, McCreary C (2011) Impact of chronic oral mucosal disease on daily life: Preliminary observations from a qualitative study. Oral Dis 17: 265-269.
- https://handbook.cochrane.org/chapter_8/8_5_the_cochrane_collaborations_tool_for_assessing_risk_of_bias.htm.
- Aswath N, Kumar STP, Jayesh SR, Manigandan T, Sarumathi T (2014) A randomized, open label, clinical study of synbiotics in patients with recurrent minor aphthous ulcers. RJPBCS 5: 1900-1905.
- Bhalang K, Thunyakitpisal P, Acemannan RN (2013) A polysaccharide extracted from aloe vera, is effective in the treatment of oral aphthous ulceration. J Altern Complement Med 19: 429-434.
- Najafi S, Mohammadzadeh M, Monsef Esfahani HMG, Rezaei N (2013) The effect of purslane in the treatment of recurrent aphthous stomatitis. Tehran Univ Med J 71: 102-108.
- Rad F, Yaghmaee RM, Khatibi R (2010) A comparative clinical trial of topical triamcinolone (Adcortyle) and a herbal solution for the treatment of minor aphthous stomatitis. Armaghane danesh 15: 191-198.
- Eslami Raveshty S, Eslami Raveshty S (2011) The effect of combining essences of Myrtus communis and Melissa officinalis in the treatment of minor aphta. ZUMS Journal 19: 77-83.
- Katti G, Divakar DD (2011) Amlexanox in the treatment of recurrent minor aphthous ulcers. Int J Dent Clin.
- Mansour G, Ouda S, Shaker A (2013) Clinical efficacy of new aloe vera- and myrrh-based oral mucoadhesive gels in the management of minor recurrent aphthous stomatitis: A randomized, double-blind, vehicle-controlled study. J Oral Pathol Med 43: 405-409.
- Jiang XW, Zhang Y, Song GD, Li FF, Peng HY, et al. (2012) Clinical evaluation of allicin oral adhesive tablets in the treatment of recurrent aphthous ulceration. Oral Surg Oral Med Oral Pathol Oral Radiol 113: 500-504.
- Raeesi V, Arbabi-Kalati F, Akbari N, Hamishekar H (2015) Comparison effectiveness of the bioadhesive paste containing licorice 5% with bioadhesive paste without drug in the management of recurrent aphthous stomatitis. Acta Medica Mediterranea. 1: 131-1335.
- Pourahmad M, Rahiminejad M, Fadaei S, Kashafi H (2010) Effects of camel thorn distillate on recurrent oral aphthous lesions. J Dtsch Dermatol Ges 8: 348-352.
- Guintu FZ, Chua AH (2013) Effectivity of guava leaves (Psidium guajava) as mouthwash for patients with aphthous ulcers. Philipp J Otolaryngol Head Neck Surg 1: 8-13.
- Deshmukh RA, Bagewadi AS (2014) Comparison of effectiveness of curcumin with triamcinolone acetonide in the gel form in treatment of minor recurrent aphthous stomatitis: A randomized clinical trial. Int J Pharm Investig 4: 138-141.
- Seyyedi SA, Sanatkhani M, Pakfetrat A, Olyaee P (2014) The therapeutic effects of chamomilla tincture mouthwash on oral aphthae: A randomized clinical trial. J Clin Exp Dent 6: e535-538.
- El-Haddad SA, Asiri FY, Al-Qahtani HH, Al-Ghmlas AS (2014) Efficacy of honey in comparison to topical corticosteroid for treatment of recurrent minor aphthous ulceration: A randomized, blind, controlled, parallel, double-center clinical trial. Quintessence Int 45: 691-701.
- He Y, Gong D, Zhu R (2014) The efficacy of kasmitad gel in the management of recurrent minor aphthous ulceration. J Kuwait Med Assoc 46: 124-129.
- Hoseinpour H, Peel SA, Rakhshandeh H, Forouzanfar A, Taheri M, et al. (2011) Evaluation of rosa damascena mouthwash in the treatment of recurrent aphthous stomatitis: A randomized, double-blinded, placebo-controlled clinical trial. Quintessence Int 42: 483-491.
- Jiang XW, Zhang Y, Zhu YL, Zhang H, Lu K, et al. (2013) Effects of berberine gelatin on recurrent aphthous stomatitis: A randomized, placebo-controlled, double-blind trial in a Chinese cohort. Oral Surg Oral Med Oral Pathol Oral Radiol 115: 212-217.
- Sukumaran V (2010) A randomized, double-blind, placebo-controlled comparative study to evaluate the efficacy of HiOra-SG gel in stomatitis. IJCP 21: 321-325.
- Ghalayani P, Zolfaghary B, Farhad AR, Tavangar A, Soleymani B (2013) The efficacy of Punica granatum extract in the management of recurrent aphthous stomatitis. J Res Pharm Pract 2: 88-92.
- Stojanovska AA, Popovska M, Muratovska I, Mitic K, Stefanovska E, et al. (2014) The rapeutic effect of proaftol in treatment of recurrent aphthous stomatitis. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 35: 195-202.
- Ali HS, Rasool BKA (2011) Propolis buccal paste in treatment of aphthous ulceration: Formulation and clinical evaluation. Asian J Pharm Clin Res 4: 29-33.
- Babaee N, Zabihi E, Mohseni S, Moghadamnia AA (2012) Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent Res J (Isfahan) 9: 381-385.
- Bechir A, Sirbu R, Pacurar M, Podariu AC, Monea M, et al. (2014) The effect of collagenic gels with marine algae extracts mixtures in the treatment of recurrent aphthous stomatitis. Revista de Chimie 65: 362-368.
- Gavanji S, Larki B (2013) Preparing of a new herbal medicine formulation for minor aphthous ulcers. IJSRIN 1: 1.
- Halim DS, Mahanani ES, Saini R, Omar M, Rubiantee bt Ibrahi N, et al. (2013) A comparison study on the effectiveness of local honey and salicylate gel for treatment of minor recurrent aphtous stomatitis. Int Med J 20: 770-772.
- Hamdy AA, Ibrahem MA (2010) Management of aphthous ulceration with topical quercetin: A randomized clinical trial. J Contemp Dent Pract 11: E009-E016.
- Khademi H, Iranmanesh P, Moeini A, Tavangar A (2014) Evaluation of the effectiveness of the iralvex gel on the recurrent aphthous stomatitis management. Int Sch Res Notices 2014: 175378.
- Liu X, Guan X, Chen R, Hua H, Liu Y, et al. (2012) Repurposing of yunnan baiyao as an alternative therapy for minor recurrent aphthous stomatitis. Evid Based Complement Alternat Med 2012: 284620.
- Romero-Cerecero O, Zamilpa A, Tortoriello J (2015) Pilot study that evaluated the clinical effectiveness and safety of a phytopharmaceutical elaborated with an extract of Ageratina pichinchensis in patients with minor recurrent aphthous stomatitis. J Ethnopharmacol 173: 225-230.
- Mohammady MTC, Abdoli S (2014) Risk of bias in randomized controlled trials published in Iranian nursing and midwifery journals in 2010. Ira J Epidemiol 9: 24-36.
- Hamedi S, Sadeghpour O, Shamsardekani MR, Amin G, Hajighasemali D, et al. (2016) The Most common herbs to cure the most common oral disease: Stomatitis recurrent aphthous ulcer (RAU). Iran Red Crescent Med J 18: e21694.
- Ranganath SP, Pai A (2016) Is optimal management of recurrent aphthous stomatitis possible? A reality check. J Clin Diagn Res 10: Ze08-ze13.