Suryakant Kengar1*, Dattatray Thorat2 and Jaywant Jadhav3
1Department of Zoology, Yashwantrao Chavan College of Science, Karad, Maharashtra, India
2Department of Zoology, Balasaheb Desai College, Patan, Maharashtra, India
3Cell Biology Section, Department of Zoology, Shivaji University, Kolhapur, Maharashtra, India
*Corresponding Author:
Suryakant Kengar
Department of Zoology, Yashwantrao Chavan College of Science
Karad, Maharashtra
India
Tel: +919850571346;
E-mail: sbkengar@ymail.com
Received date: April 03, 2017; Accepted date: May 09, 2017; Published date: May 12, 2017
Citation: Kengar S, Thorat D, Jadhav J (2017) Nephroprotective Effect of Amaranthus spinosus Root Extract in Carbon Tetrachloride Induced Histological Toxicity in Male Albino Rat. Int J Drug Dev & Res 9: 05-07
Keywords
Amaranthus spinosus; CCl4; Nephrotoxicity; Histopathological; Oxidative damage
Introduction
Oxidative stress in cells and tissues result from the increased generation of free radicals or reactive oxygen species (ROS) [1,2]. Free radicals are produced in cells by cellular metabolism and by exogenous agents that induce numerous pathological complications [3-5]. Carbon tetrachloride (CCl4), a commonly used hepatotoxin in experimental models to induce oxidative stress. It is known to induce free radicals in liver and kidney and cause cellular damage [6-8]. The kidneys are responsible for elimination of unmodified drugs and metabolites. The certain metabolites accumulated in the kidney induce nephrotoxicity. It reportedly gets distributed at higher concentrations in the kidney leading to renal damage [9-11]. It shows a high affinity to the cortex which contains cytochrome P-450 predominantly [12,13]. The ingredients with antioxidant activity that acts as a free radical scavenger have a great potential to protect oxidative damage.
Amaranthus spinosus Linn. (Family Amaranthaceae), commonly known as “pig weed” is known for its several pharmacological properties. It is used as anti-inflammatory, anti-malarial, antibacterial, anti-diuretic, antiviral and hepatic disorders. The studies on A. spinosus have been carried out by various researchers and a wide spectrum of its pharmacological actions have been explored which may include antinephritic, antidiabetic, antitumor, analgesic, antimicrobial, anti-inflammatory, spasmolytic, bronchodilator, hepato-protective, spermatogenic, antifertility, antimalarial, antioxidant properties, etc. [14-20]. Phytochemical investigations revealed the presence of several active constituents like alkaloids, flavonoids, glycosides, phenolic acids, steroids, amino acids, terpenoids, lipids, saponin, betalain, b-sitosterol, stigmasterol, linoleic acid, rutin, catechuic tannins and carotenoids [16,21,22].
The reported hepatoprotective, antioxidative, antidiabetic activity of the root extract of A. spinosus prompted us to investigate protective efficacy of ethanolic root extracts of this plant in CCl4 induced oxidative damage in kidney of male albino rat through histological assessment.
Materials and Methods
Plant material and preparation of extracts
The fresh root of A. spinosus were obtained from the rural area of the Karad (Dist. Satara, MH, India) and were properly identified. The roots were washed, shed dried and powdered mechanically with standard method. The root powder was extracted with ethanol. The dissolved extracts were filtered through Whatman filter paper (No.1). Supernatants of each extracts were evaporated at 60°C to dryness. After evaporation extracts were collected and stored at 4°C.
To study the effects of ethanolic root extracts of A. spinosus on kidney different doses viz; 150, 300 and 450 mg/kg body wt. were used.
Experimental design for hepatocurative activity
Male albino rats, Rattus norvegicus (Wistar strain) weighing 180- 220 g were used for the experiments. The rats were acclimatized under standard laboratory condition and were fed with standard pellet diet (Amrit feeds, Sangli, Maharashtra, India). Animals were randomly allocated into six groups of six each as per various treatments given as follows:
a) Group I: Normal rats without any treatment.
b) Group II: Acute hepatotoxicity induced by administration of 3.0 ml CCl4 /kg body wt/day for 15 days subcutaneously.
c) Group III: 3.0 ml CCl4/kg body wt/day for 15 days (sc)+50 mg dose of A. spinosus root extract/kg body wt. for 15 days simultaneously.
d) Group IV: 3.0 ml CCl4/kg body wt/day for 15 days (sc)+150 mg dose of A. spinosus root extract/kg body wt. for 15 days simultaneously.
e) Group V: 3.0 ml CCl4/kg body wt/day for 15 days (sc)+300 mg dose of A. spinosus root extract/kg body wt. for 15 days simultaneously.
f) Group VI: 3.0 ml CCl4/kg body wt/day for 15 days (sc)+450 mg dose of A. spinosus root extract/kg body wt. for 15 days simultaneously.
g) At the end of experimental period, all the animals were sacrificed and tissues were taken for histopathological studies.
Histological preparation
The kidney tissues were dissected out immediately after sacrifice animal and processed for histological assessment. Tissues were fixed in CAF and processed for paraffin sectioning as per routine micro technique. The specimens were washed and dehydrated in ascending grades of alcohol. Then cleared and embedded in paraffin. The paraffin sections of 5 mm thickness were cut and further processed and stained with hematoxylin-eosin. The sections were observed under light microscope for histopathological alterations in kidney.
Results and Discussion
Several studies have showed that plants possessing free radical scavenging properties could be playing an important role in protection of oxidative damages in different organs such as liver, kidney, brain; caused by environmental and chemical toxicants through metabolic activation to highly reactive substances such as free radicals. In the present study, A. spinosus roots extracts in ethanol was tested for protective efficacy against CCl4 induced kidney damage in male albino rat.
The histological observation of the kidney of normal rat revealed the normal cortex and medulla region. It showed normal glomeruli (g) with Bowman’s capsule (bc). Endothelial cells were normal. The proximal tubules (pt) with distinct lumina filled with anatomizing brush border and distal tubules (dt) was normal with clear lumina. The stromal and interstitial tissues can be differentiated. Administration of 3.0 ml CCl4/kg body wt per day for 15 days resulted marked deleterious histological changes. It showed significant glomerular and tubular degenerations. Glomerular congestion has been observed with disintegrated Bowman’s capsules. Vacuolar and degenerative changes were also evident in endothelial lining of glomerular tuft and in epithelial lining of renal tubules. Swollen proximal tubules and dilated Bowman’s capsule was observed. Swollen collecting dusts, distinct intertubular connective tissue and thickening of collecting tubules are indicator of constant irritation (Figure 1) [23].
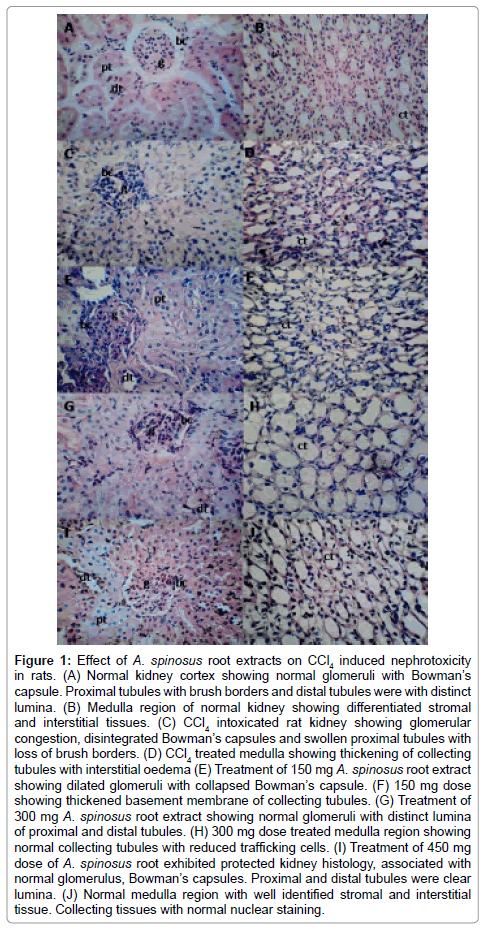
Figure 1: Effect of A. spinosus root extracts on CCl4 induced nephrotoxicity in rats. (A) Normal kidney cortex showing normal glomeruli with Bowman’s capsule. Proximal tubules with brush borders and distal tubules were with distinct lumina. (B) Medulla region of normal kidney showing differentiated stromal and interstitial tissues. (C) CCl4 intoxicated rat kidney showing glomerular congestion, disintegrated Bowman’s capsules and swollen proximal tubules with loss of brush borders. (D) CCl4 treated medulla showing thickening of collecting tubules with interstitial oedema (E) Treatment of 150 mg A. spinosus root extract showing dilated glomeruli with collapsed Bowman’s capsule. (F) 150 mg dose showing thickened basement membrane of collecting tubules. (G) Treatment of 300 mg A. spinosus root extract showing normal glomeruli with distinct lumina of proximal and distal tubules. (H) 300 mg dose treated medulla region showing normal collecting tubules with reduced trafficking cells. (I) Treatment of 450 mg dose of A. spinosus root exhibited protected kidney histology, associated with normal glomerulus, Bowman’s capsules. Proximal and distal tubules were clear lumina. (J) Normal medulla region with well identified stromal and interstitial tissue. Collecting tissues with normal nuclear staining.
For the protective experimental schedule three doses 150, 300 and 450 mg/kg body wt. were tested. These doses (PO) were initiated simultaneously with the CCl4 administration. Treatment of 150 mg/kg body wt. dose given for 15 days resulted in collapsed lumina. Bowman’s capsule was disintegrated. Luminas of proximal and distal tubules were not differentiated due to loss of brush border of proximal tubules. Basement membranes of tubules was thickened. Administration of 300 mg/kg body wt. dose showed normal glomerulus. Distinct lumina of proximal and distal tubules. Collecting tubules were observed with reduced trafficking cells. Treatment of 450 mg/kg body wt. dose of ethanol extracts of A. spinosus root protected normal morphology of the kidney and showed normal architecture of the kidney. It showed normal glomerulus, cortex and medulla. It seems to be related with the increased dose. It is associated with normal proximal tubules with distinct brush borders, distal tubules and collecting tubules. The collecting tubules were normal with reduced trafficking cells. Connecting tissue with normal nuclear staining. These structural improvements were also observed with the tissues treated with 300 mg dose but were comparatively in reduced form.
The observed nephroprotective effect of A. spinosus roots honey might have been due to the presence of phenolic and flavonoidal contents. The active ingredients contents in A. spinosus roots in this dose only can control the oxidation. The active ingredients can control the oxidation and peroxidation process of lipids, resulting in reduction of tissue damage and help to continue the blood flow in the kidney and improvement of kidney functionality [24-26].
The results indicated that ethanolic extract of A. spinosus root at 450 mg dose protects the kidney from the histological alterations caused by oxidative damage; by improving disrupted metabolisms and antioxidant defense against CCl4 induced oxidative damage in 15 days’ protective experimental schedule. The findings support the therapeutic use of A. spinosus in ailments caused by oxidative and altered metabolisms.
19510
References
- Mylonas C, Kouretas D (1999) Lipid peroxidation and tissue damage. In vivo 13: 295-309.
- Sumida Y, Niki E, Naito Y, Yoshikawa T (2013) Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res 47: 869-880.
- Singh PP, Fazana M, Roy A, SharmaP (2009) Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J Clin Biochem24: 324-342.
- Bhattacharya A, Chattopadhyaya R, Mitra S, Crowe SE (2014) Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94: 329-354.
- Trnkova L, Drsata J,Bousova I (2015) Oxidation as an important factor of protein damage: Implications for Maillard reaction. J Bioscience 40: 419-439.
- Roullier CH (1963) Experimental toxic injury of the liver. New York, USA, pp: 335-443.
- Ozturk F, Ucar M, Ozturk C, Vard N, Batcıoglu K (2003) Carbon tetrachloride induced nephrotoxicity and protective effect of betaine in sprague-dawley rats. Urology 62: 353-356.
- Sanzgiri UY, Srivatsan V, Muralidhara S, Dallas CE, Bruckner JV (1997) Uptake, distribution, and elimination of carbon tetrachloride in rat tissues following inhalation and ingestion exposures. Toxicol and Applied Pharmacol 143: 120-129.
- Sener G, Sehirli AO, Altunbas HZ, Ersoy Y, Paskaloglu K, et al. (2002) Melatonin protects against gentamicin induced nephrotoxicity in rats. J Pineal Res32: 231-236.
- Teli P, Jadhav J, Thorat D, Kanase A (2014) Primary response of graded doses of mica derived Ayurvedic drug, Abhrak bhasma and silicon dioxide on liver and kidney histology in male albino rat. J Pharma Res 8: 877-883.
- Jaramillo-juarez F, Rodriguez-Vazquez ML, Rincon-Sanchez AR, Martinez C, Ortiz GG, et al. (2008) Acute renal failure induced by carbon tetrachloride in rats with hepatic cirrhosis. Ann Hepatol 7:331-338.
- Abraham P, Wilfred G, Cathrine SP (1999) Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clinica Chimica Acta289: 177-179.
- Samy RP, Ignacimuthu S, Raja DP (1999) Pre-liminary screening of ethnomedical plants from India. J Ethnopharmacol66: 235-240.
- Srivastava R, Shukla YN, Kumar S (1998)Chemistry, Pharmacology and Botany of Boerhavia diffusa. a review. J Med Aroma Plant Sci20: 762-767.
- Harsha Vardhana S (2011) In vitro antibacterial activity of Amaranthus spinosus root extracts. Pharmacophore 2: 266-270.
- Berghofer E, Schoenlechner R (2002) Grain amaranth. In: Pseudocereals and Less Common Cereals. Grain Properties and Utilization Potential. Belton PS, Taylor Jr N (eds.), NewYork, pp: 219-260.
- Zeashan H, Amresh G, Singh S, Rao CV (2008) Hepatoprotective activity of Amaranthus spinosus in experimental rats. Food Chem Toxicol 46: 3417-3421.
- Zeashan H, Amresh G, Singh S, Rao CV (2009) Hepatoprotective and antioxidant activity of Amaranthus spinosus against CCl4 induced toxicity. J Ethnopharmacol 125: 364-366.
- Zeashan H, Amresh G, Singh S, Rao CV (2009) Antidiarrheal and antiulcer activity of Amaranthus spinosus in experimental animals. Pharm Biol 47: 932-939.
- Azhar-ul-Haq M, Afza N, Khan SB, Muhammad P (2006) Coumaroyl adenosine and lignin glycoside from Amaranthus spinosus Linn. Pol J Chem 80: 259-263.
- Blunden G, Yang M, Janicsak MI, Carabot-Cuervo A (1999) Betaine distribution in the Amaranthaceae. Biochem Syst Ecol 27: 87-92.
- Majno G, Joris I (2004) Cells, Tissues and Disease.Principals of General Pathology. 2ndedn. Oxford University Press, New York, USA.
- Rafieian-Kopaei M, Baradaran A, Rafieian M (2013) Oxidative stress and the paradoxical effects of antioxidants. J Res Med Sci 18: 629.
- Rafieian-Kopaei M, Baradaran A, Rafieian M (2013) Plants antioxidants: from laboratory to clinic. J Nephropathol 2: 152-153.
- Baradaran A, Nasri H, Nematbakhsh M, Rafieian- Kopaei M (2014) Antioxidant activity and preventive effect of aqueous leaf extract of Aloe vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter 165: 7-11.







