Case Report - (2024) Volume 15, Issue 4
Neurofibromatosis type 1, case report of surgical treatment of cervical c2 kissing neurofibromas
Marcel Sincari1*,
Francisco Cabrita2,
Eduardo Mendes3 and
Mark Sincari4
1Department of Surgery, Centro Hospitalar, Tondela-Viseu, Portugal
2Department of Surgery, CUF Viseu Hospital, Viseu, Portugal
3Department of Surgery, Centro Hospitalar, Ortopedy, Viseu, Portugal
4Department of Medicine, University of Coimbra, FMUC, Coimbra, Portugal
*Correspondence:
Marcel Sincari, Department of Surgery, Centro Hospitalar, Tondela-Viseu,
Portugal,
Email:
Received: 23-Jul-2024, Manuscript No. ipjnn-24-15068;
Editor assigned: 25-Jul-2024, Pre QC No. P-15068;
Reviewed: 14-Aug-2024, QC No. Q-15068;
Revised: 20-Aug-2024, Manuscript No. R-15068;
Published:
26-Aug-2024
Abstract
Neurofibromatosis type 1 is an autosomal dominant genetic syndrome
associated with numerous neoplastic and non-neoplastic manifestations
affecting a variety of organ systems, including skin, eye, nervous system,
skeleton and endocrine and gastrointestinal tract. The diagnosis and
clinical course of this disease is associated with a variety of different
challenges. Postoperative management of surgically treated cases is
even more difficult. We present a case of a young lady with NF1 with
kissing NFBs of C2 root with severe cervical spine compression and
signs of myelopathy treated surgically. The postoperative period was
practically the follow up of complications and addressing them. The
final result is very good and the overall follow up was 5 years.
Keywords
Neurofibroma; Myelopathy; Laminectomy
Acronyms
NF1: Neurofibromatosis type 1; NFBs: Neurofibromas;
ACDF: Anterior Cervical Decompression and Fusion; CSF: Cerebrospinal
Fluid
Introduction
The earliest descriptions of NF can be seen in Egypt
papyrus of Ebers in 1500 before Christ, as well in artistic
crops like Hellenistic statuette dated in 323 before Christ.
More recent artistic descriptions are mosaic illustration
of Ulise Aldrovandis, Monstrum Historia in 1592. The
Quasimodo in the Victor Hugo´s novel Notre-Dame
de Paris, published in 1832, is though that has NF [1].
One year later after Victor Hugo had published his
Notre-Dame de Paris, in 1833, Friedrich Daniel von
Recklinghausen (Virchow´s pupil) was born and had the
first official interpretation of NF1 as an illness in 1882
on a report “On multiple cutaneous fibromas and their
relationship with multiple neuromas” he was the first to
name the tumors neurofibromas [2]. NF1 is estimated to
occur in 1:3,000 live births. It is extremely variable in its
clinical presentation from generalized expression of the
disease (90% of the cases) [3] to localized neurofibromas
or café-au-lait spots [4]. According to another sources NF1
incidence is estimated of 1 in 2,700 and a prevalence of 1
in 4,500 [3].
NF1 represents monogenic disorders presenting a
pattern of autosomal dominant inheritance, with a high
rate of de novo mutations of 42%. These mutations are
believed to be responsible for the sporadic appearance of
NF1 [5]. Penetrance is complete or, at least, nearly so before
the age of 5 years, but expressivity differs greatly between
affected individuals, even within the same consanguinity.
There is no predilection for sex, race, or ethnicity [6,7].
NF1 is the most common tumor predisposition syndrome
inherited in an autosomal dominant (100% penetrance)
fashion with a wide variety of expressivity [8]. The lifetime
risk of malignancy in individuals with NF1 is estimated to
be 59.6% [9], justifying the lifelong surveillance of patients
with NF1 [10].
The diagnostic criteria for NF1 were defined in 1987
by the National Institute of Health Criteria Consensus
Conference:
• Six or more spots of “cafe au lait” type more than 5
mm in diameter,
• Two or more neurofibromas or one plexiform
neurofibroma,
• Freckles or skin discolorations in the places
inaccessible for light (armpits, groins, pubic region),
• Two or more Lisch nodules on the iris,
• Characteristic skeletal changes,
• A first-degree relative suffering from NF13.
At least two criteria must be met for clinical diagnosis [11].
Characteristic skeletal changes of NF1 include
congenital pseudarthrosis of the tibia, scoliosis, sphenoid
wing dysplasia, rib penciling, and gracile bones [12].
Scoliosis can be considered the most common type of spinal
deformity that it is present in 10% to 71% of cases [13].
According to another authors, spine deformities in NF1
including scoliosis, kyphosis and atlantoaxial instability are
in 2-36% of the patients [14].
Malignant transformation leads to the development of
such tumors as malignant peripheral nerve sheath tumor,
or malignant triton tumor. Other soft tissue sarcomas, like
rhabdomyosarcoma, as well as other malignancies such as
juvenile myelomonocytic leukemia or pheochromocytoma
are also encountered in NF1 patients with increased
frequency comparing to general population [15,16]. In
spite of this, in 1992, the participants of a joint World
Health Organization and National Neurofibromatosis
Foundation meeting declared that “although some clinicians have advocated routine screening scans for all
patients with NF1, the utility of such screening has not
been conclusively demonstrated” [17]. According to some
sources, taking into account the rarity of complications
that are usually symptomatic and easily detected during
the clinical follow-up, screening investigations are not
recommended [15-18]. Positron emission tomography/
computed tomography are useful for the detection of
malignant transformation of tumors in NF1 patients [19].
Case Presentation
34 years old lady, with NF1 diagnosed since the age of
18 years, admitted because of unstable gait. MRI revealed
bilateral kissing C2 roots tumors with severe bilateral
compression of the spinal cord, spinal cord is squeezed in
between C2 bilateral tumors and C3 root left sided tumor Fig. 1. She was operated on, the extra and intradural growing
tumors were removed through C2, C3 laminectomy with
subsequent dural reconstruction with nuchal ligament
harvest during the approach. In the postoperative period
she developed a huge, tense, subaponeurotic CSF collection Fig. 2. solved only after inserting the ventriculoperitoneal
shunt with the use of navigation, because of small ventricles Fig. 3. Two years after the neurofibroma removal she was
bothered by neck pain and fixed position in anterior flexion
of the head. X-Ray, MRI diagnosed post laminectomy
regional kyphosis C2-C3 with anterior luxation. She was
selected for circumferential arthrodesis (ACDF C3-C4,
C4-C5, posterior mass lateral fixation C2-C6 on the right
side, C2, C3, C5, C6 on the left side) with good recovery
and pain relief Fig. 4. Three years after she started to be
bothered by upper limb paresthesia and MRI revealed
initial cervical syringomyelia. One year later her gait was
progressively more unstable and MRI showed significant syringomyelia progression Fig. 5. And next surgery was
performed: siringopleural shunt insertion trough upper
thoracic laminectomy Th1, Th2 and unilateral intralaminar
fixation C7-Th3 was performed at the end of the surgery Fig 6. The decision to fix C7-Th3 with intralaminar
unilateral screws was dictated by previous experience with
cervical instability and weak muscles of the patient. The
cranial hand of the proximal T shaped shunt is placed inside the syrinx cavity and the caudal part was positioned
in the subdural space Fig. 7. The distal part was inserted
in the pleural space through the intercostal space. The
postoperative period was uneventful, the gait improved
significantly. The patient follow-up until now is 5 years,
she is doing well with very few unspecific complaints. She
is seen annually by neurosurgeon and neurologist Fig. 8.
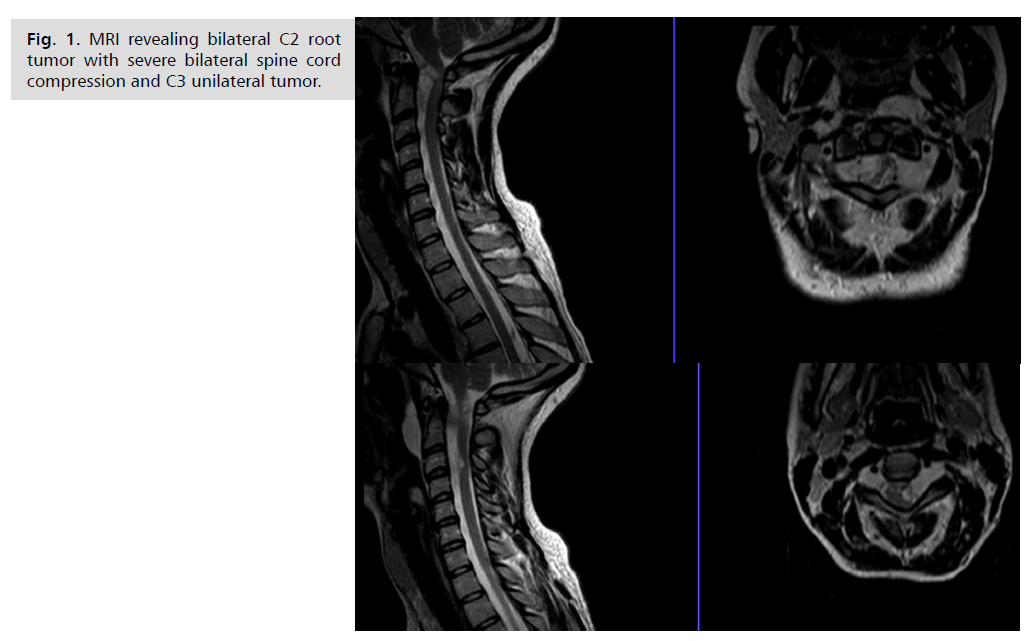
Fig. 1: MRI revealing bilateral C2 root
tumor with severe bilateral spine cord
compression and C3 unilateral tumor.
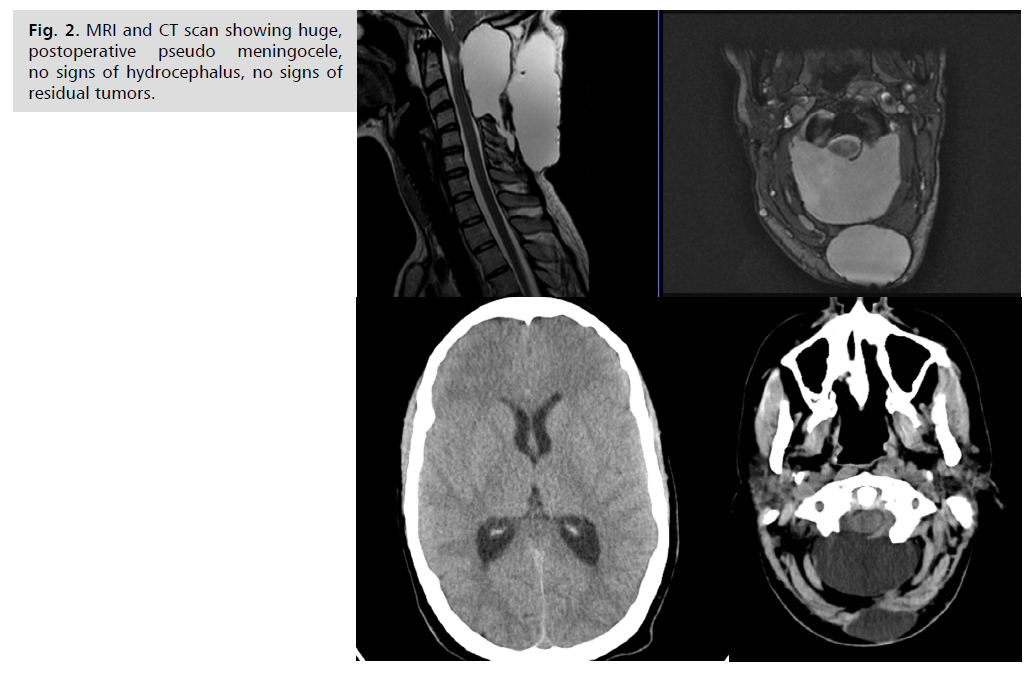
Fig. 2: MRI and CT scan showing huge,
postoperative pseudo meningocele,
no signs of hydrocephalus, no signs of
residual tumors.

Fig. 3: Serial CT scan after ventriculoperitoneal
shunting, meningocele diminished
on serial CT scan.

Fig. 4: MRI revealing upper cervical post
laminectomy regional kyphosis, syringomyelia,
no signs of meningocele. X-Ray
before and after 360-degree arthrodesis.
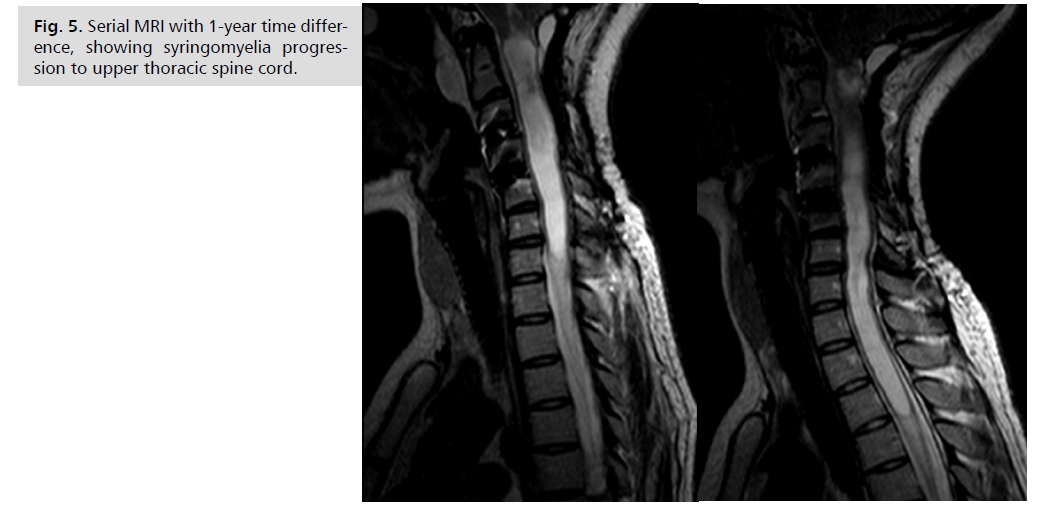
Fig. 5: Serial MRI with 1-year time difference,
showing syringomyelia progression
to upper thoracic spine cord.
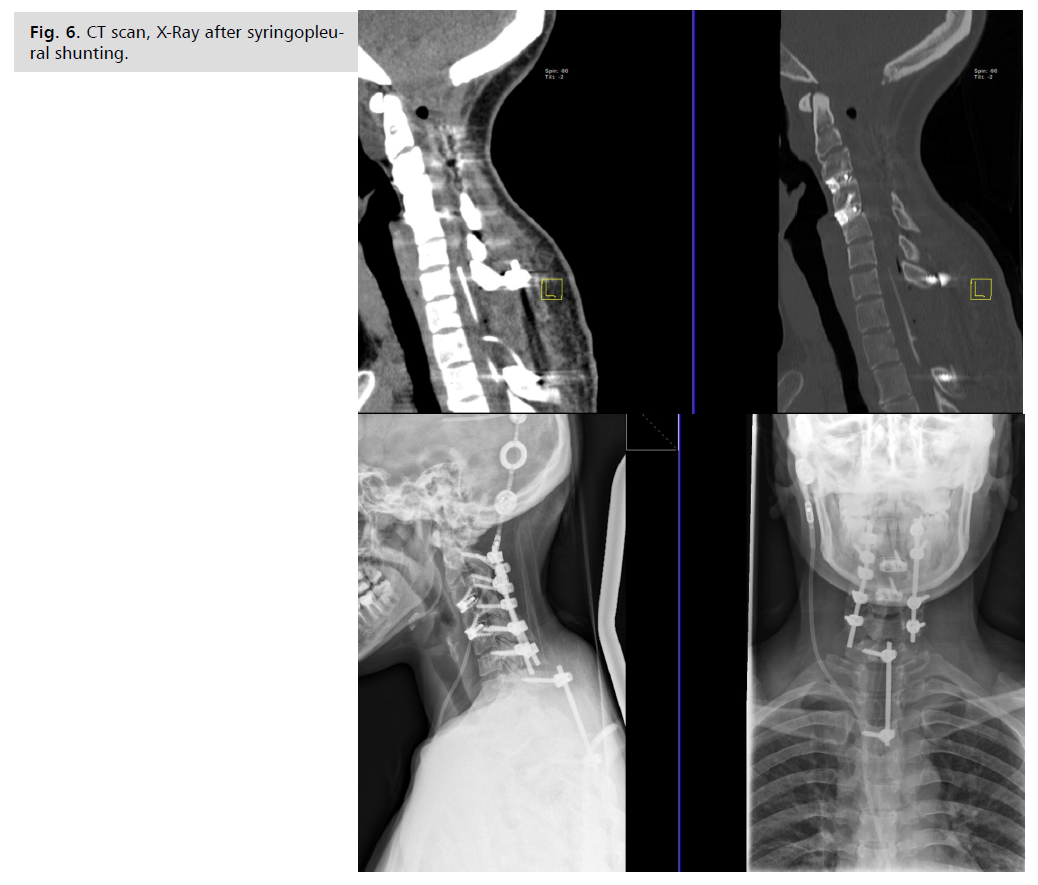
Fig. 6: CT scan, X-Ray after syringopleural
shunting.
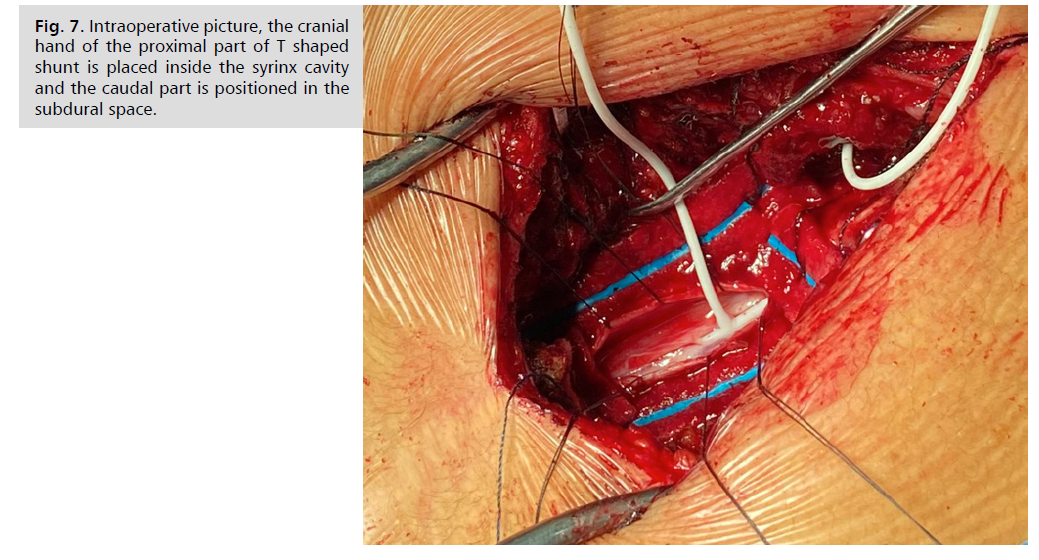
Fig. 7: Intraoperative picture, the cranial
hand of the proximal part of T shaped
shunt is placed inside the syrinx cavity
and the caudal part is positioned in the
subdural space.
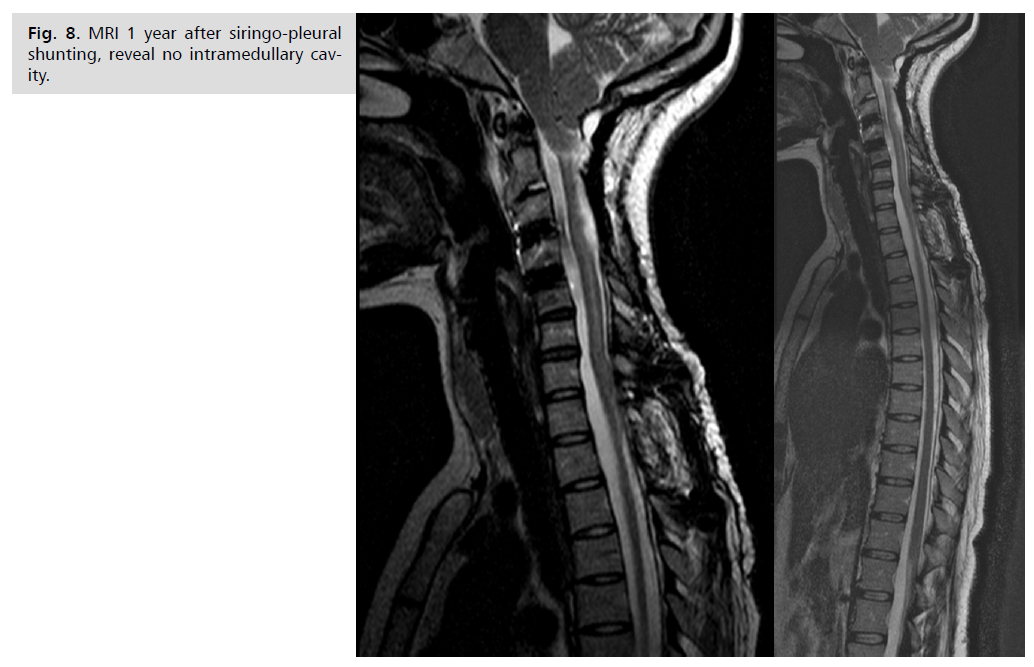
Fig. 8: MRI 1 year after siringo-pleural
shunting, reveal no intramedullary cavity.
Discussion
The most common tumor found in NF1 patients are
NFBs, followed by plexiform NFBs, malignant peripheric
nerve sheath tumors, and glial tumors [20]. Contrary to
NF2, the tumors are less frequently found in the intradural
spinal compartment in NF1 (less than10% of the cases),
with most of them located laterally to the neuroforamina
[21].
Sometimes, like in the case described here, NFBs are
found bilaterally in two nerve roots at the same spinal
level, resulting in significant cord compression. These
lesions are known as “kissing neurofibromas”, generally
with progressive myelopathy in the cervical spine, usually
with surgical indication to relieve cord compression. In
the literature is mentioned that decompressive procedures
in the cervical spine in NF1 patients will ultimately cause
some degree of deformity, even in adult patients [22], like
what happened in our case.
The best treatment of malignant peripheric nerve tumors
is complete excision. Adjuvant radiation and chemotherapy
have unclear success rates. Neurofibromatosis-1
patients with MPNSTs have a 5-year survival rate of
approximately 21% [23]. Hydrocephalus may be an
unexpected postsurgical sequela in these patients [24]. The
subaponeurotic CSF collection after removal of C2 tumors
in our opinion is like external hydrocephalus that regressed
only after ventriculoperitoneal shunting [25-27].
Conclusion
Despite our wide knowledge concerning NF1 there are still numerous clinical and surgical challenges to be solved.
Surgery is the principal line of treatment for neurofibromas,
but comes with a high recurrence rate after partial removal
of large plexiform neurofibromas. In the case of NF1-
related tumors, there is no consensus with regard to the
treatment strategy due to the multiple pathways involved
in the growth of NF1 tumors. More relevant spinal
complications of NF1 include pseudarthrosis, bleeding,
hematoma formation. Progression of the deformity may
also be observed, as well as, dural leaks. Based on a group
of 22 patients treated surgically, in retrospective analysis,
it was concluded that early stabilization of the cervical
spine prevents late deformity of the patients with NF1. In
our case we experienced almost all possible complications,
more relevant being CSF flow disturbances, huge tense
subaponeurotic collection solved with shunting and
progressive syringomyelia, in spite of good functioning of
ventriculoperitoneal shunt, that was resolved with siringopleural
shunting. Another major complication was cervical
post-laminectomy kyphosis that could be avoided if initial
fixation would be done at the first surgery. These emphasize
that preoperative planning and meticulous follow up of the
surgically treated patient’s withnNF1 is an important issue
that improves life quality.
Funding
None.
Conflict of Interest
None.
References
- Ruggieri M, Praticò AD, Caltabiano R, et al. Early history of the different forms of neurofibromatosis from ancient Egypt to the British Empire and beyond: first descriptions, medical curiosities, misconceptions, landmarks, and the persons behind the syndromes. Am J Med Genet A. 2018; 176(3):515-50.
Google Scholar, Crossref, Indexed at
- Violante IR. The neurobiological basis of Neurofibromatosis type I: new insights into brain structure, function and neurochemistry. (Doctoral dissertation, Universidade de Coimbra (Portugal)). 2012.
Google Scholar
- Riccardi VM. Von Recklinghausen Neurofibromatosis. N Engl J Med. 1981; 305(27):1617–1627.
Google Scholar
- Roth RR, Martines R, James WD. Segmental neurofibromatosis. Arch Dermatol. 1987;123(7):917-20.
Google Scholar
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010; 152A:327-32.
Google Scholar, Crossref, Indexed at
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999; 89: 1-6.
Google Scholar, Crossref, Indexed at
- Antonio JR, Goloni-Bertollo EM, Tridico LA. Neurofibromatosis: chronological history and current issues. An Bras Dermatol. 2013; 88: 329-43.
Google Scholar, Crossref, Indexed at
- Choi J, An S, Lim SY. Current concepts of neurofibromatosis type 1: pathophysiology and treatment. Arch Craniofacial Surg. 2022;23(1):6-16.
Google Scholar, Crossref, Indexed at
- Uusitalo E, Rantanen M, Kallionpaa RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016; 34:1978-86.
Google Scholar, Crossref, Indexed at
- Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019; 103(6): 1035-54.
Google Scholar, Crossref, Indexed at
- Stumpf DA, Alksne JF, Annegers JF, et al. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988; 45(575):8.
Google Scholar, Indexed at
- Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1). Bone. 2008; 42(4):616-22.
Google Scholar, Crossref, Indexed at
- Haddadi K, Kargar Soleiman Abad S, Hashemie Amir SM, et al. Spinal Manifestations of Neurofibromatosis: An Update. Iran J Neurosurg. 2020; 6(4):169-80.
Google Scholar, Crossref, Indexed at
- Lykissas MG, Mavrogenis AF, Megaloikonomos PD, et al. Spinal deformities in neurofibromatosis type 1. Clin Cases Miner Bone Metab. 2018;15(3):348-352.
Google Scholar
- Evans DG, Salvador H, Chang VY, et al. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res. 2017; 23(12):e46-53.
Google Scholar, Crossref, Indexed at
- Bekiesińska-Figatowska M, Brągoszewska H, Duczkowski M, et al. Circle of Willis abnormalities in children with neurofibromatosis type 1. Neurol Neurochir Pol. 2014; 48(1):15-20.
Google Scholar, Crossref, Indexed at
- World Health Organization. Prevention and control of neurofibromatosis: memorandum from a joint WHO/NNFF meeting. Bull World Health Organ. (WHO). 1992:173-82.
Google Scholar, Indexed at
- Pinson S, Créange A, Barbarot S, et al. Neurofibromatosis 1: recommendations for management. Arch Pediatr. 2002; 9(1): 49-60.
Google Scholar, Crossref, Indexed at
- Moharir M, London K, Howman-Giles R, et al. Utility of positron emission tomography for tumour surveillance in children with neurofibromatosis type 1. Eur J Nucl Med Mol Imaging. 2010; 37:1309-17.
Google Scholar, Crossref, Indexed at
- Shimizu T, Lenke LG, Cerpa M, et al. Preoperative halo-gravity traction for treatment of severe adult kyphosis and scoliosis. Spine Deform. 2020; 8:85-95.
Google Scholar, Crossref, Indexed at
- Shofty B, Barzilai O, Khashan M, et al. Spinal manifestations of neurofibromatosis type 1. Childs Nerv Syst. 2020; 36(10):2401-2408.
Google Scholar, Crossref, Indexed at
- Taleb FS, Guha A, Arnold PM, et al. Surgical management of cervical spine manifestations of neurofibromatosis Type 1: long-term clinical and radiological follow-up in 22 cases. J Neurosurg Spine. 2011; 14(3):356-66.
Google Scholar, Crossref, Indexed at
- Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009; 4(5):453-63.
Google Scholar, Crossref, Indexed at
- Shofty B, Mauda-Havakuk M, Ben-Sira L, et al. Surgical management of “kissing” spinal plexiform neurofibromas in neurofibromatosis type 1 patients. World Neurosurg. 2020; 1(134):e1143-7.
Google Scholar, Crossref, Indexed at
- Trovo‐Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet 2006; 70(1):1-3.
Google Scholar, Crossref, Indexed at
- Katz D, Lazar A, Lev D. Malignant Peripheral Nerve Sheath Tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med. 2009; 11:e30.
Google Scholar, Crossref, Indexed at
- Crawford AH, Lykissas MG, Schorry EK, et al. Neurofibromatosis: etiology, commonly encountered spinal deformities, common complications and pitfalls of surgical treatment. Spine Deform. 2012; 1(1):85-94.
Google Scholar, Crossref, Indexed at













