Lamiss Mohamed AE1*, Asama Mohamed Elkady1, Mamoun Abo Shosha2 and Dareen Mohamed3
1 Department of Clinical Oncology, Tanta University Hospital, Tanta, Egypt
2 Department of Neurosurgery, El-Azhar University, Cairo, Egypt
3 Department of Pathology, Tanta University Hospital, Tanta, Egypt
*Corresponding Author:
Lamiss Mohamed AE
Department of Clinical Oncology, Tanta University Hospital, Tanta, Egypt.
Tel: 040 3337544
Fax: 040 3337544
E-mail: lamissmohamed2@yahoo.com
Received Date: June 01, 2018; Accepted Date: June 11, 2018; Published Date: June 18, 2018
Citation: Mohamed AEL, Elkady AM, Shosha MA, Mohamed D (2018) Nonmethylated MGMT as Predictive Factor in Newly Diagnosed Glioblastoma Multiforme Treated with Bevacizumab Concurrent with Radiotherapy Followed by Adjuvant Bevacizumab plus Irinotecan versus Temozolomide Concurrent with Radiotherapy Followed by Adjuvant Temozolomide. Arch Cancer Res. Vol.6 No.2:11 DOI: 10.21767/2254-6081.100177
Keywords
Temozolomide; Glioblastoma; Temozolomide; Adjuvant chemotherapy
Introduction
The most frequent primary tumor reported in adult is glioblastoma multiforme. It is responsible for 15% of all intracranial tumors and 60–75% of astrocytoma’s [1]. The duration of survival has not changed much over the past few decades (4%) [2,3]. Since the publication of EORTC trial temozolomide (TMZ)-based chemo radiation has become the standard treatment but short median survival has been reported in patients with non-methylated O6-methylguanine–DNA methyltransferase (non-methylated MGMT) [4,5]. Thus, few trials are applied to test the response GBM with non-methylated MGMT [6]. Glioblastoma multiforme is characterized by high degree of neovascularization caused by secreted vascular endothelial growth factor A (VEGF-A) [7,8]. The blood brain barrier is highly disrupted due to formation of dysfunctional new vasculature leading to focal brain edema [9]. Bevacizumab (BEV) is a monoclonal humanized antibody of the immunoglobulin G1 (IgG1) against VEGF-A was developed, inhibiting neovascularization and reducing vascular permeability then reducing focal brain edema [10]. In recurrent glioblastome multiforme, a progression free survival benefit but not overall survival benefit was reported with bevacizumab from several non-randomized trials [11,12]. As first line setting the same results were reported as improved progression free survival but not overall survival [13,14]. The most astonishing trial is Glarius trial which randomized 182 patients with glioblastome multiforme to bevacizumab concurrent with radiotherapy followed by sequential bevacizumab plus irinotecan bimonthly versus daily temozolomide concurrent radiotherapy then six cycles of temozolomide. It resulted in improvement in progression of survival not translated in improvement of overall survival [15]. Irinotecan has marginal activity in recurrent glioblastoma multiforme with progression free survival at 6 months 16% [16]. Irinotecan active metabolite (100-1000 times more active inhibitor of topoisomerse) is 7-ethyl-10-hydroxycamptothecin (SN-38). Gliomas cells can convert irinotecan to SN-38 directly which decrease proliferation with resultant apoptosis [17]. So our trial was to test the role of bevacizumab with irinotecan versus temozolomide in non-methylated MGMT newly diagnosed glioblastoma multiforme.
Patient and Methods
It was a randomized, controlled, trial in Oncology Department, Tanta University Hospital and Neurosurgery Department, al Azhar University, Cairo approved by Medical Ethic Committee of Tanta University Hospital between August 2013 and August 2015. All patients gave written informed consent. Flow chart of patients was shown in Figure 1. The study included newly diagnosed glioblastoma multiforme with age more than 18 years, eastern cooperative oncology 0-2, adequate healing of craniotomy flap, non-methylated MGMT (ratio<0.6); stable or decreasing corticosteroids within 5 days before random assignment; and adequate hematologic, hepatic, renal, and coagulation function. Patients with any of the following criteria presence of overt recent haemorrhage detected by magnetic resonance imaging with contrast; history of thromboembolic disease, bleeding diathesis, coagulopathy, stomach ulcer, gastrointestinal perforation or serious non-healing wound or fracture were excluded from the study. First the specimen was reread by pathologist in pathology department, Tanta University to confirm the glioblastoma multiforme diagnosis. After confirmation it was analysed for MGMT promoter methylation using a real-time methylationspecific polymerase chain reaction (PCR) [18,19].
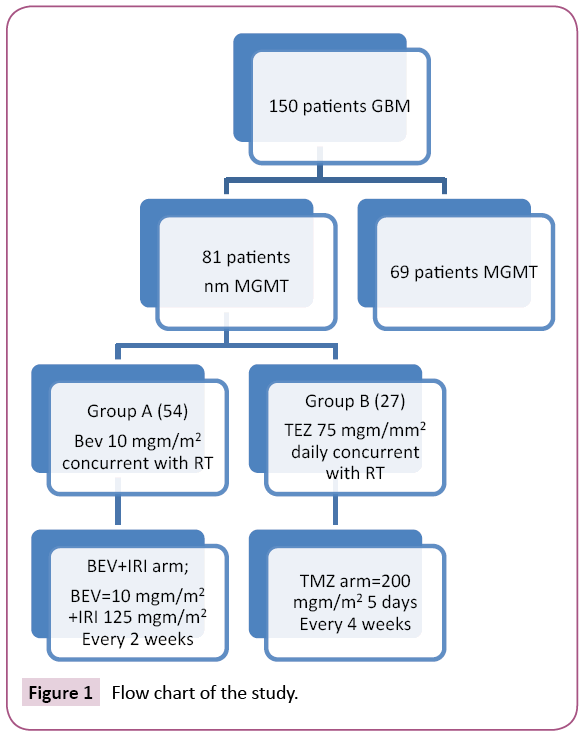
Figure 1: Flow chart of the study.
According to manufacturer's recommendations, assessing DNA methylation of the MGMT promoter region by applying methylation specific PCR is done. Firstly treat Genomic DNA (1 μg) with sodium bisulphite then the EpiTect Bisulphite Kit (Qiagen) for purification. Then the extracted DNA was amplified with published PCR primers that distinguish methylated and unmethylated DNA [18,19]. Ratio less than 0.6 is considered nm MGMT (non-methylated MGMT). Radiotherapy was given to all patients 4 weeks postoperative in the form of involved field radiotherapy (radiotherapy dose All patients received involved-field radiotherapy) (RT; 33 × 180 cGy). The patient were randomized into two groups, group A received bevacizumab (BEV) (10 mg/kg every 2 weeks) during radiotherapy, followed by maintenance BEV (10 mg/kg every 2 weeks) plus irinotecan (IRI) (125 mg/m2 every 2 weeks) until progressive disease. Patients in the group B received 75 mg/m2 daily temozolomide (TMZ) during RT followed by adjuvant chemotherapy six cycles of TMZ (200 mg/m2 once daily for 5 days every 4 weeks). In recurrence in group B; patients could receive second-line BEV+IRI.
Response and Toxicity
After two months from the end of radiotherapy evaluation of patients using neurological examination and magnetic resonance imaging with contrast were done. Response evaluation was done using RECIST version 1.1 [20]. After radiotherapy progressive disease was diagnosed only if the tumor progressed outside the radiation field. Then evaluation was done every three months until death. Neurological examination, corticosteroid use and MRI brain with contrast were used to evaluate progressive disease. Determination of progressive disease (PD) was based on MRI, clinical assessment, and corticosteroid use [21]. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) [22].
End Points and Statistical Analysis
The primary end point was the progression free survival using Kaplan Meier version 21 [23]. Correlation with different prognostic factor including age, sex, performance status, extent of resection, and type of treatment received. Secondary survival end points were overall response and OS. Survival analyses were done using Kaplan-Meier method, log-rank test for group comparisons.
Results
Patient Characteristics
Between august 2013 and August 2015, one hundred and fifty patients with newly diagnosed GBM were analysed MGMT promotor methylation status. Of them 81 (54%) and 69 (46%) patients had an nm MGMT ratio less than 0.6, and methylated MGMT respectively. The non-methylated group were then randomized in ratio 2:1 to group A (BEV+IRI arm; n=54), group B (TMZ arm, n=27); baseline characteristics listed in Table 1.
| Patient Characteristics |
Total (81) |
BEV + IRI arm (54) |
TMZ arm (27) |
P-value |
| Age |
50yr |
52yr |
50yr |
0.993 |
| Median Range |
26-80 + 13.20 |
26-80 + 12.89 |
26-78 + 13.99 |
| Sex |
Male |
64 (79%) |
42 (77.8%) |
22 (81.5%) |
0.7 |
| Female |
17 (21%) |
12 (22.2%) |
5 (18.5%) |
| Performance status |
0-1 |
57 (70.4%) |
38 (70.4%) |
19 (70.4%) |
1 |
| 2 |
24 (29.6%) |
16 (29.6%) |
8 (29.6%) |
| Extent of resection |
Biopsy |
33 (40.7%) |
28 (51.9%) |
5 (18.5%) |
0.015* |
| Partial resection |
43 (53.1%) |
23 (42.6%) |
20 (74.1%) |
| Complete resection |
5 (6.2 %) |
3 (5.6%) |
2 (7.4%) |
Table 1: Patient characteristics.
Treatment
In the group A (BEV+IRI arm), number of biweekly BEV was from 5-80 cycles with median of 22 biweekly course. Three year progression free survival with BEV+IRI versus TMZ was 81.5%, 38.6% respectively which was statistically significant (p-value=0.001) with median survival was 21 and 17 in group A and B respectively (Figure 2). According to female sex, three progression free survival was 83.3%, 80% in the BEV+IRI and TMZ group respectively; also according to male sex three progression free was 81.6%, 27.3% in the BEV+IRI and TMZ group respectively which was statistically significant (p-value=0.001). As regard age, three year progression free survival was 92.4% versus 32% for patients aged less than 50 years in group A and B respectively ; also in patients aged more than 50 yrs, it was higher in group A versus group B (70.1% versus 46.3% respectively and this was statistically sigficant (p-value=0.001). In patients with performance status 0-1, three progression free survival was 82.1%, 27.3% in the BEV+IRI and TMZ group respectively; also in performance status 2 it was 79.6% in group A versus 50% 50% which was statistically significant (p-value=0.001). According to resection, three year PFS biopsy, partial resection, and total resection it was 86.5%, 75.7, 100% versus 80%, 33.3, 0% in group A and B respectively which was statistically significant (p-value=0.029). Multivariate analysis of progression free survival revealed the prognostic factors with statistical significance were performance status (0.043), degree of resection (0.032) and type of treatment (0.003). Three year overall survival was 74.9% and 41.9% in relation to line of treatment BEV+IRI versus TMZ respectively which was statistically significant (p-value=0.011) with median survival for group A was 21.5 months versus 19 for group B (Figure 3). According to female sex, three overall survival was 71.4%, 60% in the BEV+IRI and TMZ group respectively ; also according to male sex Three overall survival was 76.2%, 37.1% in the BEV+IRI and TMZ group respectively which was statistically significant (p-value=0.007) As regard age, three year OS was 72.8% versus 45.2% for patients aged less than 50 years in group A and B respectively ; also in patients aged more than 50 yrs it was higher in group A versus group B (78.4% versus 37% respectively and this was statistically significant (p-value=0.015). In patients with performance status 0-1,three overall survival was 72.6%, 38.8% in the BEV+IRI and TMZ group respectively; while in patients with performance status 2 it was 80.8% versus 53.6% in group A and B respectively which was statistically significant (p-value=0.017). According to resection three year OS biopsy, partial resection, and total resection were 86%, 62%, 100% versus 80%, 37.7%, 0% in group A and B respectively which was statistically not significant (p-value=0.213). Multivariate analysis of overall survival revealed the prognostic factors with statistical significance was type of treatment (0.037). Complete response, partial response and overall response were higher in group A (OAR=81.5%) versus group B (OAR=44.4%) which was statistically significant (P-value=0.001) as shown in Table 2. Correlation of overall response with line of treatment according to different prognostic factors, the factors with statistical significance were male sex (p-value=0.001), age less than or equal 50 yrs (p-value=0.001), performance status 0-1 (p-value=0.003) and partial resection (p-value=0.001) (Table 3). In BEV+IRI group three cerebral haemorrhages occurred of which one was fatal. In TMZ group, high-grade hematotoxicity reported in 5 (18.5%) patients. Grade 3 nausea, vomiting and diarrhoea were higher with BEV+IRI [20 (37.03%) patients] than with TMZ [5 (15%) patients], may be attributed to IRI. Grade 4 vomiting and diarrhoea was reported only in IRI group [3 patients (5.6%)].
| Treatment |
|
Response |
Total |
P-value |
| CR |
PR |
SD |
PD |
| BEV + IRI arm |
Count |
19 |
22 |
6 |
4 |
51 |
0.001* |
| % within treatment |
37.30% |
43.10% |
11.80% |
7.80% |
100.00% |
| % within response |
100.00% |
64.70% |
35.30% |
50.00% |
65.40% |
| % of Total |
24.40% |
28.20% |
7.70% |
5.10% |
65.40% |
| TMZ arm |
Count |
0 |
12 |
11 |
4 |
27 |
| % within treatment |
0.00% |
44.40% |
40.70% |
14.80% |
100.00% |
| % within response |
0.00% |
35.30% |
64.70% |
50.00% |
34.60% |
| % of Total |
0.00% |
15.40% |
14.10% |
5.10% |
34.60% |
| BEV + IRI |
OAR |
44 (81.5%) |
| Non responsive |
10 (18.5%) |
| TMZ |
OAR |
12 (44.4 %) |
| Non responsive |
15 (55.6 %) |
Table 2: Treatment response according to line of treatment.
| Patient characteristics |
Overall response |
BEV + IRI |
TMZ |
P value |
| N (%) |
N (%) |
| Sex |
Male |
OAR |
35 (83.3%) |
9 (40.1%) |
0.001* |
| Non responsive |
7 (16.7%) |
13 (59.1%) |
| Female |
OAR |
9 (75%) |
3 (60%) |
0.536 |
| Non responsive |
3 (25%) |
2 (40%) |
| Age |
< 50 yr or equal |
OAR |
24 (88.9%) |
6 (40 %) |
0.001* |
| Non responsive |
3 (11.1%) |
9 (60%) |
| 50 yr |
OAR |
20 (74.1%) |
6 (50%) |
0.536 |
| Non responsive |
7 (25.9%) |
6 (50%) |
| Performance status |
0-1 |
OAR |
31 (81.6%) |
8 (42.1%) |
0.003* |
| Non responsive |
7 (18.4%) |
11 (57.9%) |
| 2 |
OAR |
13 (81.3%) |
4 (50%) |
0.112 |
| Non responsive |
3 (18.7%) |
4 (60%) |
| Extent of resection |
Biopsy |
OAR |
22 (78.6%) |
3 (60%) |
0.372 |
| Non responsive |
6 (21.4%) |
2 (40%) |
| Partial resection |
OAR |
20 (87%) |
8 (40%) |
0.001* |
| Non responsive |
3 (13%) |
12 (60%) |
| Complete resection |
OAR |
2 (66.6%) |
1 (50%) |
0.709 |
| Non responsive |
1 (33.3%) |
1 (50%) |
Table 3: Correlation of overall response with line of treatment according to different prognostic factors.
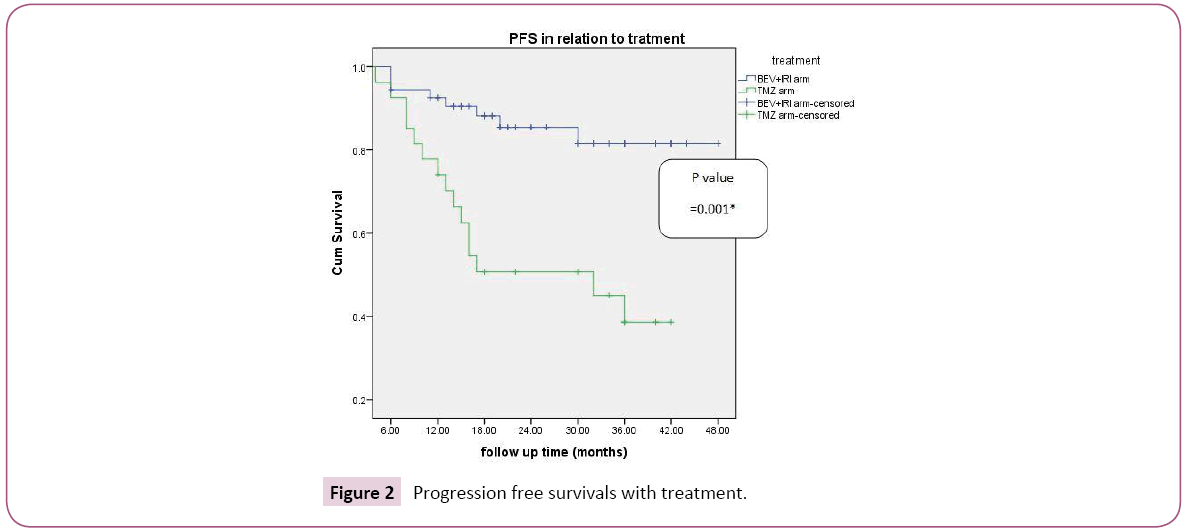
Figure 2: Progression free survivals with treatment.
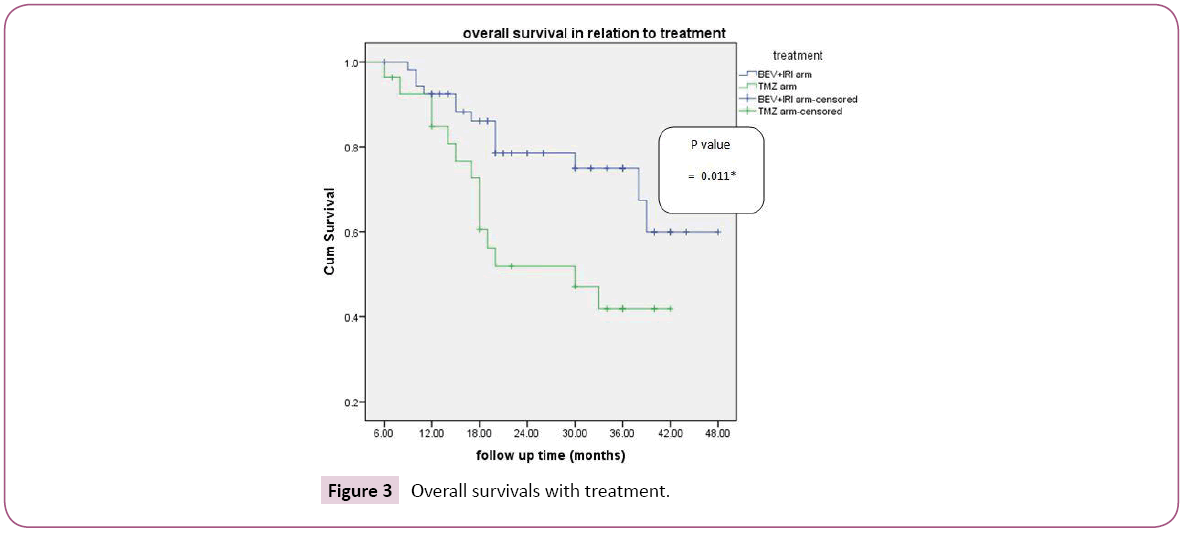
Figure 3: Overall survivals with treatment.
Discussion
Newly diagnosed non methylated MGMT (nm MGMT) GBM treated with BEV+IRI had better overall response, progression free survival and overall survival than standard TMZ. This may provide a good alternative to standard TMZ and this may be good option for patients with nm MGMT. The prognostic factors with statically significance in correlating overall response with line of treatment were male sex (p-value=0.001), age less than or equal 50 yrs (p-value=0.001), performance status 0-1 (p-value= 0.003) and partial resection (p-value=0.001). As regard progression free survival was higher BEV+IRI versus TMZ 81.5%, 38.6% respectively which was statistically significant (p-value=0.00) and this was constant with that reported by Glarius study [15]. The PFS was prolonged by BEV was similar to that reported by the larger phase III AVAglio and RTOG 0825 trials [24] despite different line of treatment as patient received during radiotherapy. In univariate analysis with progression free survival, all prognostic factors including age, sex, performance status and treatment were statistically significant but in multivariate analysis, the prognostic factors with statistical significance were performance status, degree of resection and type of treatment. In our trial the progression free survival was translated into improvement in overall survival in contrast to as reported by Glarius [15], Aglio and RTOG 0825 trials in which improvement in PFS was not translated into improvement in overall survival [15,24]. The progression free survival was not translated into improvement in OS may be attributed to anti edema effect and pseudo progressive effect of BEV and not true anti neoplastic effect. In univariate analysis of overall survival with different prognostic factors, age, sex performance status and line of treatment were significant but in multivariate analysis, type of treatment was only statistically significant prognostic factor Grade 3 or higher adverse events were higher in the bevacizumab+IRI group than in TMZ group, this may be explained by higher incidence of hypertension in first group. However haematological toxicity was higher in TMZ group but nausea, vomiting and diarrhoea higher in BEV+IRI may be attributable to irinotecan side effect and this was constant with that reported by Glarius trial [15].
Conclusion
It may be concluded that due to improvement in both PFS and OS a new strategic plan for patients with nm MGMT newly diagnosed glioblastome multiforme should search for other alternatives for standard concurrent temozolomide and adjuvant temozolomide in newly diagnosed glioblastome multiforme of which BEV+IRI appeared to be promising.
Conflict of Interest
Authors declared that they had no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Tanta university hospital and informed consent was taken from patients included in this study.
22913
References
- Rogers TW, Toor G, Drummond K, Love C, Field K, et al. (2018) The 2016 revision of the WHO classification of central nervous system tumours: Retrospective application to à cohort of diffuse gliomas. J Neurooncol 137: 1-9.
- Smoll NR, Schaller K, Gautschi OP (2013) Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci 20:670-675.
- Nizamutdinov D, Stock EM, Dandashi JA, Vasquez EA, Mao Y, et al. (2018) Prognostication of Survival Outcomes in Patients Diagnosed with Glioblastoma. World Neurosurg 109: 67-74.
- Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, et al. (2009) Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: A long-term analysis. Tumori 95:191-197.
- Stupp R, Hegi ME, Mason WP, Van den Bent MJ, Taphoorn MJ, et al. (2009) European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: A 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459-466.
- Lalezari S, Chou AP, Tran A, Solis OE, Khanlou N, et al. (2013) A combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastome outcome. Neuro Oncol 15:370-381.
- Jhaveri N, Chen TC, Hofman FM (2016) Tumor vasculature and glioma stem cells: Contributions to glioma progression. Cancer Lett 380:545-551.
- Burger MC, Breuer S, Cieplik HC, Harter PN, Franz K, et al. (2017) Bevacizumab for Patients with Recurrent Multifocal Glioblastomas. Int J Mol Sci 18: 2469.
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, et al. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 91:1071-1121.
- Brandes AA, Finocchiaro G, Zagonel V, Reni M, Fabi A, et al. (2017) Early tumor shrinkage as a survival predictor in patients with recurrent glioblastome treated with bevacizumab in the AVAREG randomized phase II study. Oncotarget 8:55575-55581.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, et al. (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol27: 4733-4740.
- Burger MC, Breuer S, Cieplik HC, Harter PN, Franz K, et al. (2017) Bevacizumab for Patients with Recurrent Multifocal Glioblastomas. Int J Mol Sci 18: E2469.
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma.N Engl J Med 370:709-722.
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, et al. (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699-708.
- Herrlinger U, Schafer N, Steinbach JP, Weyerbrock A, Hau P, et al. (2016) Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J Clin Oncol 34: 1611-1619.
- Metz MZ, Gutova M, Lacey SF, Abramyants Y, Vo T, et al. (2013) Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: implications for clinical use. Stem Cells Transl Med 2:983-992.
- Rogers TW, Toor G, Drummond K, Love C, Field K, et al. (2018) The 2016 revision of the WHO classification of central nervous system tumours: Retrospective application to à cohort of diffuse gliomas. J Neurooncol 137: 1-9.
- Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, et al. (2006) North American Brain Tumor Consortium: A phase 2 trial of irinotectan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol 8:189-93.
- Smoll NR, Schaller K, Gautschi OP (2013) Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci 20:670-675.
- Shah N, Schroeder B, Cobbs C (2015) MGMT methylation in glioblastome: A tale of the tail. Neuro Oncol 17:167-168.
- Nizamutdinov D, Stock EM, Dandashi JA, Vasquez EA, Mao Y, et al. (2018) Prognostication of Survival Outcomes in Patients Diagnosed with Glioblastoma. World Neurosurg 109: 67-74.
- Christians A, Hartmann C, Benner A, Meyer J, Von Deimling A, et al. (2012) Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastome. PLoSOne 7: 33449.
- Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, et al. (2009) Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: A long-term analysis. Tumori 95:191-197.
- Schwartz LH, Litiere S, De Vries E, Ford R, Gwyther S, et al. (2016) RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 62:132-137.
- Stupp R, Hegi ME, Mason WP, Van den Bent MJ, Taphoorn MJ, et al. (2009) European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: A 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459-466.
- Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. MJ Clin Oncol 8:1277-1280.
- Lalezari S, Chou AP, Tran A, Solis OE, Khanlou N, et al. (2013) A combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastome outcome. Neuro Oncol 15:370-381.
- Kluetz PG, Chingos DT, Basch EM, Mitchell SA (2016) Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ 35:67.
- Jhaveri N, Chen TC, Hofman FM (2016) Tumor vasculature and glioma stem cells: Contributions to glioma progression. Cancer Lett 380:545-551.
- Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Amer Statist Assn 53: 457-481.
- Burger MC, Breuer S, Cieplik HC, Harter PN, Franz K, et al. (2017) Bevacizumab for Patients with Recurrent Multifocal Glioblastomas. Int J Mol Sci 18: 2469.
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709-722.
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, et al. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 91:1071-1121.
- Brandes AA, Finocchiaro G, Zagonel V, Reni M, Fabi A, et al. (2017) Early tumor shrinkage as a survival predictor in patients with recurrent glioblastome treated with bevacizumab in the AVAREG randomized phase II study. Oncotarget 8:55575-55581.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, et al. (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol27: 4733-4740.
- Burger MC, Breuer S, Cieplik HC, Harter PN, Franz K, et al. (2017) Bevacizumab for Patients with Recurrent Multifocal Glioblastomas. Int J Mol Sci 18: E2469.
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma.N Engl J Med 370:709-722.
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, et al. (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699-708.
- Herrlinger U, Schafer N, Steinbach JP, Weyerbrock A, Hau P, et al. (2016) Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J Clin Oncol 34: 1611-1619.
- Metz MZ, Gutova M, Lacey SF, Abramyants Y, Vo T, et al. (2013) Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: implications for clinical use. Stem Cells Transl Med 2:983-992.
- Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, et al. (2006) North American Brain Tumor Consortium: A phase 2 trial of irinotectan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol 8:189-93.
- Shah N, Schroeder B, Cobbs C (2015) MGMT methylation in glioblastome: A tale of the tail. Neuro Oncol 17:167-168.
- Christians A, Hartmann C, Benner A, Meyer J, Von Deimling A, et al. (2012) Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastome. PLoSOne 7: 33449.
- Schwartz LH, Litiere S, De Vries E, Ford R, Gwyther S, et al. (2016) RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 62:132-137.
- Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. MJ Clin Oncol 8:1277-1280.
- Kluetz PG, Chingos DT, Basch EM, Mitchell SA (2016) Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ 35:67.
- Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Amer Statist Assn 53: 457-481.
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709-722.








