Key words
|
| |
| Piperidone, Isomeric Alcohols, NMR, DPPH. |
| |
INTRODUCTION
|
| |
| During a fairly recent 10-year period, several thousand piperidine compounds have been mentioned in clinical and preclinical studies [1]. Besides the interesting structural features, these compounds are also of pharmaceutical interest as they exhibit a wide range of biological activities [2]. Nevertheless, the variety of functionality and substitution patterns found in piperidine targets and the widely accepted concept that the biological properties of piperidines are highly dependent on the type and location of substituents on the heterocyclic ring [3]. Piperidine derivatives are found to possess pharmacological activity and form an essential part of the molecular structures of important drugs such as raloxifene and minoxidil [4].Selective inhibition of a number of enzymes involved in the binding and processing of glycoproteins has rendered piperidine alkaloids as important tools in the study of biochemical pathways [5]. Piperidones are somewhat less prominent, but often they serve a role as advanced intermediates prior to their conversion to piperidines [6]. Hydroxylated piperidine alkaloids are frequently found in living systems and display a wide range of biological activities due to their ability to imitate carbohydrates in a variety of enzymatic processes [7]. In many different disciplines antioxidants become more interested in new compounds, either synthesized or obtained from natural sources that could provide active components to prevent or reduce the impact of oxidative stress on cell [8]. Antioxidants are extensively studied for their capacity to protect organism and cell from damage that are induced by oxidative stress. Oxidative damages play a significantly pathological role in human diseases. Cancer, emphysema, cirrhosis, atherosclerosis and arthritis have all been correlated with oxidative damage. Also, excessive generation of reactive oxygen species (ROS) induced by various stimuli and which exceeds the antioxidant capacity of the organism leads to variety of pathophysiological processes such as inflammation, diabetes, genotoxicity and cancer[9].In the present study wide range applications of radical scavenging and antioxidant activities of newly synthesized compounds were investigated using DPPH method. |
| |
CHEMISTRY
|
| |
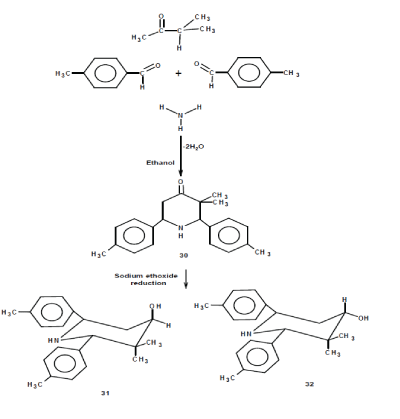
| Synthesis and isomerization of piperidine-4-ols have been described by Noller and Balasubramanian [10, 17]. |
| |
 |
| |
BIOLOGY
|
| |
| The in vitro antioxidant activity of the synthesized compounds was evaluated by DPPH assay method. [11] |
| |
STATISTICAL ANALYSIS
|
| |
| Statistical significance of difference between the mean activities of the subgroups of compounds was determined by utilizing ANOVA for variables with normal distributions. |
| |
RESULT AND DISCUSSION
|
| |
| IR spectra |
| |
| The prominent peaks around 3376, 3425 cm-1 in the IR spectra are attributed to υOH, mode in 2and 3 respectively shows that C=O in piperidone 1 was reduced into secondary isomeric alcohols –CH-OH values are reproduced in Table 2. |
| |
| 1 H NMR |
| |
| The signals in the 1H NMR spectra were assigned based on their positions, multiplicities and integrals. Due to syn-1,3-diaxial interaction with protons at C(2) and C(6) equatorial proton at C(4) in axial alcohol 2resonated at 4.02ppm whereas axial proton at C(4) in equatorial alcohol 3 resonated at 3.87 ppm. It confirms that H(4) in axial alcohol 2 gets deshielded than in equatorial alcohol 3. |
| |
| 13C NMR |
| |
| In 13 C NMR the above theme gets reversed chemical shift of C(4) in equatorial alcohol 3 shifted to downfield range than in axial alcohol 2. |
| |
| DPPH Radical scavenging activity |
| |
| The model of scavenging the stable DPPH radical model is a widely used method to evaluate antioxidant activities in a relatively short time compared with other methods. The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen donating ability [12]. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [13]. The reduction capability of DPPH radicals was determined by decrease in its absorbance at 517 nm induced by antioxidants. The absorption maximum of a stable DPPH radical in ethanol was at 517 nm. The decrease in absorbance of DPPH radical caused by antioxidants, because of reaction between antioxidant molecules and radical, progresses, which result in the scavenging of the radical by hydrogen donation. It is visually noticeable as a discoloration from purple to yellow. Hence, DPPH is usually used as a substrate to evaluate antioxidative activity of antioxidants [14].Data of antioxidant evaluation were expressed as mean ± standard error of the mean (n=3) and independent student’s t-test was used to determine the level of significance (P <0.05).In this study antiradical activities of synthesized compounds 1-3 and standard antioxidant such as α-tocopherol were determine by using DPPH method. |
| |
| |
| The inhibitory effect of synthesized compounds 1-3 on DPPH radical was showed in figure 1.With the increase of concentration, the scavenging effect also increased. It was reported that reducing power serves as a significant indicator of antioxidant activity for a compound. The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radicals chain by donating a hydrogen atom [15].Data reveal that the reducing power of piperidone1 was stronger than its isomeric alcohols. Among the isomeric alcohols, equatorial alcohol 3 shows much better antiradical activity than axial alcohol 2.As a result of 1,3-diaxial interaction axial alcohol 2 shows lower antiradical scavenging effect to DPPH radical. These results are evident from Table.1 |
| |
EXPERIMENTAL
|
| |
|
CHEMISTRY
|
| |
| IR spectra |
| |
| Infrared spectra were recorded on a NICOLET AVATAR 360 FT-IR spectrometer. The sample was mixed with KBr and the pellet technique was adopted to record the spectra. |
| |
| Proton spectra were recorded on a AMX 400 NMR instrument operating at 400 MHz. Samples were prepared by dissolving 10 mg of the substance in 0.5 mL of CDCl3 containing 1% TMS. The spectral parameters used are, number of transients, 32; number of data points, 32 K; spectral sweep width 4000 Hz. |
| |
| 13C NMR spectra |
| |
| Proton decoupled 13C NMR spectra were recorded on a AMX 400 NMR instrument operating at 100 MHz. Solutions for the measurement of spectra were prepared by dissolving 0.5 g of the sample in 2.5 mL of CDCl3 containing a few drops TMS as internal reference. The solvent chloroform-d also provided the internal field frequency lock signal. The spectral parameters used are, number of transients 5000; number of data points 32K; pulse width 6 s (45°), spectral sweep width, 2200 Hz. |
| |
|
GENERAL PROCEDURE FOR SYNTHESIS OF COMPOUND
|
| |
| Dry ammonium acetate (0.5 mole) was dissolved in 50 ml of distilled ethanol was mixed with 4- methylbenzaldehyde (1 mole) and ethylisopropylketone (0.5mol) in distilled ethanol [10] was heated and cooled. After cooling the viscous liquid was dissolved in ether(50ml) and shaken with concentrated HCl (5ml)resulting the 3,3-dimethylr( 2),C(6)-ditolylpiperdin-4-one was precipitated as its hydrochloride then the precipitate was filtered and washed with 50ml of Ether: Ethanol mixture(4:1) to remove colored impurities. By adding aqueous ammonia and acetone the base was liberated from ethanolic solution. It was recrystillized from ethanol. 1HNMR: 7.34[m.H(ortho)], 7.18[m,H(meta)], 3.80(s,H(6)), 3.79(s.H(2)),2.93(t,H5a), 2.46(d,H5e), 2.36(s,p-CH3), 1.93(s,NH), 1.21(s,EqCH3), 0.96(s,Ax CH3). |
| |
| 3CNMR:213.16(C(4)), 140.23(ipso), 137.32(Ph), 69.28(C(2)), 61.33(C(6)), 49.91(C(3)), 47.25(C(5)), 21.07(p-CH3), 20.41(EqCH3), 19.98(Ax CH3). |
| |
|
GENERAL PROCEDURE FOR SYNTHESIS OF COMPOUNDS 2 AND3.
|
| |
| The 4-hydroxy piperdines 2 and 3 were prepared from the corresponding piperdin-4-one 1 by adopting the general procedure [16, 17]. About (0.5mol) of Piperdin-4-one 1 in distilled ethanol was refluxed with (1mol) of liquid Na at 60oC for 7hrs.Reaction mixture was subjected to Co-TLC to check the formation of isomeric alcohols. After the formation reaction mixture was poured into acidified ice water. The crude was separated by ether separation and these isomeric alcohols were separated by column chromatography using neutral alumina as adsorbent and benzene: ethyl acetate (90:10) as eluent. |
| |
| 2.1HNMR:7.38[m.H(ortho)], 7.18[m,H(meta)], 4.02(dd,H(4)), 3.80(s,H(6)), 3.78(s.H(2)), 2.92(t,H5a),2. 45(dd,H5e), 2.35(d,p-CH3), 1.58(s,NH), 1.20(s,EqCH3), 0.95(s,Ax CH3). |
| |
| 13CNMR:140.25(ipso), 137.29(Ph), 69.29C(4), 61.32C(2), 59.92C(6), 49.90C(3), 47.25C(5), 21.01(p- CH3), 20.38(EqCH3), 19.96(Ax CH3). |
| |
| 3.1HNMR:7.36[m.H(ortho)], 7.15[m,H(meta)], 3.87(dd,H(4)), 3.62(s,H(6)), 3.54(t,H(2)), H(5a),2.34(d,H5e), 1.80(t,p-CH3), 1.42(s,NH), 0.94(s,EqCH3), 0.88(s,Ax CH3). |
| |
| 13CNMR:141.65(ipso),136.75(Ph),76.72C(4),69.24C( 2),60.10C(6), 39.65C(3), 39.48C(5), 23.97(p-CH3), 21.02(EqCH3), 19.98(Ax CH3). |
| |
|
FREE RADICAL SCAVENGING ACTIVITY
|
| |
| Free radical scavenging activity of compounds was measured by DPPH, using the method of Blois [11]. Briefly, 0.1 mM solution of DPPH in ethanol was prepared, and this solution (1 mL) was added to sample solutions in CHCl3 (3 mL) at different concentrations (1-10 mg/mL). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min.Then the absorbance was measured at 517 nm in a spectrophometer. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The DPPH Concentration (mM) in the reaction medium was calculated from the following calibration curve and determined by linear regression (R: 0.997): Absorbance = 0.0003xDPPH. – 0.0174. The capability to scavenge the DPPH radical was calculated using the following equation: DPPH. Scavenging effect (%) = (A0 – A1/A0) x 100 Where, A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the samples or standards. |
| |
CONCULSION
|
| |
| In vitro antioxidant evaluation by DPPH assay concludes that piperidone exhibit strong antioxidant activity by electron donating mechanism (i.e.) it donate unshared pair of electrons on carbonyl oxygen atom. Reduction of C=O into –OH resulted in a decreased scavenging activity of isomeric alcohols compared to piperidone. Order of Antioxidant Activity: 1>3>2. |
| |
ACKNOWLEDGEMENT
|
| |
| One of the author N.Karthik convey his sincere thanks to Dr.P.Rajasekar, Senior Lecturer and Dr. Johanna Rajkumar, Professor & Head, Department of Biotechnology, Rajalakshmi Engineering College, Chennai, India for providing necessary chemicals and instruments to evaluate Antioxidant Activity. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
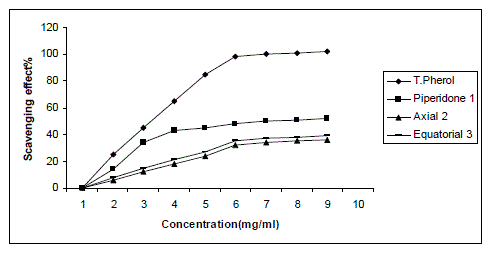 |
| Figure 1 |
|
| |








