Key words
|
| |
| Floating Microspheres, Solvent Evaporation method, Stavudine, Gastric residence time |
| |
INTRODUCTION
|
| |
| New drug delivery technologies are revolutionizing the drug discovery; development and creating R&D focused pharmaceutical industries to increase the momentum of global advancements [1]. In this regard controlled drug delivery systems have many benefits, which includes improved therapy by increasing the efficacy and gastrointestinal transit time, increased patient compliance through decreased dosing frequency and convenient routes of administration and improved site specific delivery to reduce unwanted adverse effects [2]. Gastro-retentive floating microspheres are low-density systems that have sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period. As the system floats over gastric contents, the drug is released slowly at desired rate resulting in increased gastric retention with reduced fluctuations in plasma drug concentration [3]. A wide range of microencapsulation technique has been developed to date. The selection of the technique depends upon on the nature of the polymer, the drug, and the intended use. The physicochemical parameters to be considered during the preparation of microspheres include Particle size distribution, ratio of drug to polymer, entrapment/ encapsulation efficiency. Among the various methods developed for formulation of microspheres, the solvent evaporation method has gained much attention due to its ease of fabrication without compromising the activity of drug [4] .Stavudine (D4T, thymidine) is the FDA-approved drug for clinical use for the treatment of HIV infection, AIDS and AIDS-related conditions either alone or in combination with other antiviral agents. Stavudine is typically administered orally as a capsule and an oral solution. The virustatic drug has a very short half-life (1.30 h). However patients receiving stavudine develop neuropathy and lactic acidosis. The side effects of stavudine are dosedependent and a reduction of the total administered dose reduces the severity of the toxicity [5] .The objective of the present investigation was to prepare the controlled release, microspheres of stavudine by improving biological half-life and entrapment efficiency. |
| |
MATERIALS AND METHODS
|
| |
|
Materials
|
| |
| Stavudine as a gift sample was obtained from Aurbindo Pharmaceuticals, Hyderabad, Ethyl Cellulose (Central Drug house Pvt Ltd New Delhi), Dibutyl pthalate (Ranbaxy Laboratories Ltd Chemical division SAS Nagar), Dichlromethane (Qualigens fine Chemicals Mumbai), Rectified Sprit (Hathin Faridabad Haryana). All the reagents and solvents used were of analytical grade satisfying pharmacopoeial standards. |
| |
|
Preparation of Microspheres by solvent evaporation technique
|
| |
| The polymers were dissolved in the organic solvent and the drug was added in it. This mixture was poured of water containing 0.01% Tween 80 and stirred for 20 mins. The microspheres were filtered using vacuum filtration assembly and dried [6] . |
| |
| A series of microspheres trial batches were prepared using DCM and Ethanol as organic phase, polymers such as Ethyl cellulose (EC) and Dibutyl phthalate (DB) and mixture of water and tween 80 as aqueous phase. Batches A1-A4 was prepared using Ethyl cellulose and Dibutyl phthalate in varying ratios of 1:1, 1:2, 1:3 and 1:4 respectively. In order to optimize the formulation, concentration of aqueous phase was fixed to 500ml and varying ratios of polymers were taken as shown in Table 1. The microspheres were prepared by solvent evaporation technique. |
| |
| The prepared floating microspheres were collected and weighed. The measured weight was divided by total amount of all nonvolatile components, which were used for the preparation of microspheres. The % yield was calculated using following formula [7] . |
| |
| % Yield = (Actual weight of product / Total weight of excipient and drug) x 100 |
| |
|
Particle Size Analysis
|
| |
| Measurements of the particle size distribution of microspheres were carried out with an optical microscope. Stage micrometer was used to calculate calibration factor. The particle size was calculated by multiplying the number of division of the ocular disc occupied by the particle with calibration factor. Fifty randomly chosen spheres were taken to measure their individual size [8] . |
| |
|
% Encapsulation Efficiency
|
| |
| 100 mg of Stavudine loaded microspheres were accurately weighed. They were dissolved in minimal amount of dichloromethane and the drug was extracted into pH 7.4 buffer by evaporating dichloromethane. The volume was adjusted to 100 ml with buffer. The resulting solution was then suitably diluted and analyzed for drug content spectrophotometrically at 266 nm using pH 7.4 buffer as blank. And encapsulation efficiency was calculated using formula given below: |
| |
| E = Qp/Wt * 100 |
| |
| Where E is the percentage of the encapsulated drug, Qp is the quantity of drug encapsulated in microsphere in mg and Wt is the quantity of the drug added for encapsulation in mg [9] . |
| |
|
Buoyancy Percentage
|
| |
| Microspheres were spread over the surface of dissolution apparatus (Type II) filled with 900 ml 0.1 mol L–1 HCl containing 0.02% Tween 80. The medium was agitated with a paddle rotating at 100 rpm for 12 h. The floating and the settled portions of microspheres were recovered separately. The microspheres were dried and weighed. Buoyancy percentage was calculated as the ratio of the mass of the microspheres that remained floating and the total mass of the microspheres. |
| |
| Buoyancy (%) = Qf / (Qf + Qs) x 100 |
| |
| Where Qf and Qs is weight of Floating and the settled microspheres respectively [10] . |
| |
|
Flow Properties
|
| |
| The microspheres were poured till the time when upper tip of the pile surface touched the lower tip of the funnel. The tan-1 of the height of the pile / radius of its base gave the angle of repose. Microspheres were poured gently through a glass funnel into a graduated cylinder cut exactly to 10ml mark. Excess microspheres were removed using a spatula and the weight of the cylinder with pellets required for filling the cylinder volume was calculated. The cylinder was then tapped from a height of 2.0 cm until the time when there was no more decrease in the volume. Bulk Density (rb) and Tapped Density (rt) were calculated [11] . Hausner Ratio (HR) and Carr Index (IC) were calculated according to the two equations given below: |
| |
| HR= ρt/ρb |
| |
| IC = 100 (1- ρb/ρt) |
| |
|
In-vitro Drug Release Studies
|
| |
| The in vitro release of drug from microspheres were carried out for 12 hours using paddle type Dissolution rate test Apparatus USP STD containing 900 ml of dissolution medium i.e. 0.1 N HCl buffer and maintained at 37±0.5ºC and speed of agitation at 100 rpm. An accurately weight sample was responded in dissolution media. At prefixed time (every 2 hour); 10 ml of solution were withdrawn and replaced consequently. Samples were assayed spectrophotometrically for the drug content at 266 nm using Shimadzu-1700 UV Visible spectrophotometer [12] . |
| |
|
Surface Morphology
|
| |
| Scanning electron microscopy (SEM) (Philips-XL- 20) was performed to characterize the surface of formed microspheres. Microspheres were mounted directly onto the sample stub and coated with gold film (approx 200nm) under reduced pressure (0.133 Pa) [13] . |
| |
|
Differential Scanning Calorimetry
|
| |
| The DSC analysis of pure drug, drug-loaded microspheres were carried out using Shimadzu DSC 60 to evaluate any possible drug-polymer interaction. The analysis was performed at a rate 10.0°C min-1 from 20°C to 30°C temperature range under nitrogen flow of 25 ml min-1 [13] . |
| |
|
Stability Studies
|
| |
| Accelerated stability studies were carried out to assess the shelf life of a product, but the conditions of storage are harsher. The purpose of stability study is not only to characterize the degradation of a drug product but also to establish an expiration-dating period or shelf life applicable to all future batches of drug product. The samples of microspheres were kept at 400C and 75% relative humidity for 6 weeks in the stability chamber and the samples were withdrawn and analyzed at a period of 0, 2, 4 and 6 weeks. The samples were analyzed for Physical Appearance, Particle Size, % Entrapment Efficiency and In vitro Drug Release Studies [14] . |
| |
RESULTS
|
| |
| The percentage yield of floating microspheres was greater than 49 % for all the Formulations. In the floatation test, minimum 48 % remained floating and maximum for 68% at the end of 12 h (Table 2). The mean particle size of floating microspheres of formulations A1, A2, A3 and A4 is summarized in Table no. It was evident that with increase concentration of Ethyl cellulose particle size increases. |
| |
| A low to moderate encapsulation efficiency was observed in different formulation of Stavudine. As it was evident from the results (Table 3), drug loading increases as the polymer concentration increases. Batch A1 had showed higher entrapment efficiency of 58% as compare to other batches. |
| |
| Angle of repose, Hausner ratio, and Carr’s index were determined to predict flowability. A higher Hausner ratio indicates greater cohesion between particles while a high Carr index is indicative of the tendency to form bridges. As reported in Literature. tanO value of less than 20 shows excellent flow properties while tanO of value less than 40 shows reasonable flow properties Angle of repose for stavudine loaded microspheres for batches A1-A4 was found to show reasonably good flow. The findings were supported by Carr’s (compressibility) index, which was < 20 and Hausner ratio indicating good flow characteristics (Table 4). |
| |
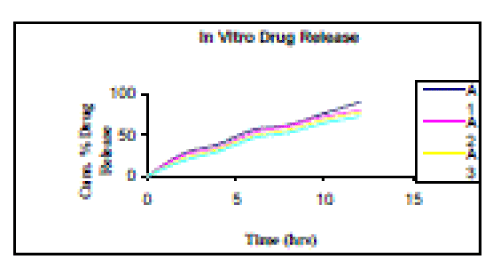
| In vitro drug release from microspheres was sustained upto 12 hours. Different formulation showed different degree of release. The cumulative release of stavudine significantly decreased with increasing ethylcellulose concentration. The increased density of the polymer matrix at higher concentrations results in an increased diffusional pathlength. This may decrease the overall drug release from the polymer matrix. The drug release was found to be maximum in case of Batch A1, which was formulated using Ethyl Cellulose and Dibutyl phthalate polymer combination in ratio of 1:1. The Cumulative % drug release for batches A1, A2, A3 and A4 was found to be 89.7, 79.8, 77.6 and 72.2% respectively at end of 12-hour percent. Among all formulation batches A1 showed best drug release as shown in Figure 4 as in this formulation maximum release of Stavudine was there in a sustained manner with constant fashion over extended period of time (after 12). |
| |
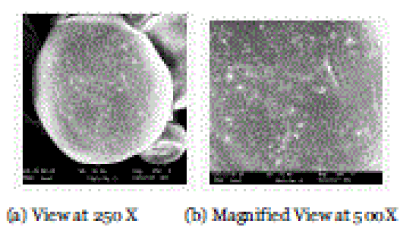
| SEM images of Batch A1 indicated that the prepared microspheres are spherical with smooth surface; distinct pores are evident on the surface of microspheres, which will be responsible for the release. The photomicrographs also showed presence of loose crystals of drug on the surface of few microspheres. |
| |
| DSC studies were performed on pure drug and drugloaded microspheres for batchA1 as shown in figure 3. Stavudine exhibits a sharp endothermic peak at 172.88 °C as shown in Figure, which corresponds to its melting point. The peak of the drug did not appear in the thermogram of any type of drug-loaded microspheres as shown in Figure. It may indicate that the drug was uniformly dispersed at the molecular level in the microspheres. |
| |
| During the Accelerated stability studies no significant change was observed in particle size and entrapment efficiency was found to decrease from 58% to 54.8%. The Cumulative % drug release from the microspheres of Batch A1 was decreased from 89.7 to 79.8%, over a period of 6 weeks at accelerated conditions. |
| |
CONCLUSION
|
| |
| The stavudine loaded microspheres were prepared by solvent evaporation method using novel polymeric combination of varied ratios of Dibutyl phthalate with EC. The prepared microspheres were free flowing and discrete. The % encapsulation efficiency was low in all formulations. The DSC suggested no drug polymer interaction during encapsulation process. The drug release was studied up to 12 hours and results indicates that release from ethylcellulose and dibutyl phthalate loaded microspheres in ratio of 1:1 was maximum. |
| |
Conflict of Interest Declaration
|
| |
| I hereby declare that I have no competing interests. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









