Caleb English, James F. Williams, Booker T. King, Leopoldo C. Cancio and Rodney K Chan*
Clinical Division and Burn Center, US Army Institute of Surgical Research, 3650 Chambers Pass, Fort Sam Houston, TX 78234, USA
*Corresponding Author:
Rodney K. Chan
M.D, FACS, FRCSC, San Antonio Military Medical Center 3851 Roger Brooke Drive
Fort Sam Houston, TX 78234
USA
Tel: 210-539-8511
Fax: 210-916-9190
E-mail: rodney.k.chan.ctr@mail.mil
Received date: December 16, 2015 Accepted date: December 24, 2015 Published date: December 31, 2016
Citation: English C, Williams JF, King BT. Objective Burn Assessment Using Laser Doppler Imaging and Indocyanine Green Videoangiography to Evaluate the Effect of Hyperbaric Oxygen Treatment: A Case Report. Ann Clin Lab Res. 2015, 3:4.
Keywords
Indocyanine green angiography, Hyperbaric oxygen therapy, Laser Doppler imaging, Burn depth assessment, Burn treatment
Introduction
Several technologies have aided the clinician in burn wound depth determination for treatment purposes; among these are laser Doppler imaging (LDI) and indocyanine green videoangiography (ICG-VA) [1-3].
LDI enables the study of perfusion of tissue by detecting movement of red blood cells in the microcirculation and thus reporting the integrity of dermal capillaries. When laser light is directed at the tissue, some is reflected off moving structures (e.g. red blood cells), undergoes a frequency shift from the Doppler effect, is detected by the photodetector, and is used to create a two dimensional image which is usually color coded for ease and speed of interpretation [4]. Clinical judgment of surgeons using indicators including appearance, blanching, and pin-prick testing has historically shown to be 60-80% accurate [2,5-7], but LDI has demonstrated the ability to determine the need for excision with accuracy approaching 100% several days in advance of clinical decision [8].
Indocyanine green (ICG) is a heavily protein-bound molecule which undergoes rapid plasma clearance by the liver with excretion into bile [9]. ICG is useful in videoangiography (VA) due to the observation that ICG absorbs and emits near-infrared light [9]. ICG-VA has been used extensively to assess tissue perfusion, especially in plastic surgery [10-12]. Recently, some authors have suggested refining it as a method to determine burn depth [1]. Since burn wound depth and severity is dependent on damage to microvascular dermal circulation, ICG-VA proves useful for determining depth and monitoring improvement.
Hyperbaric oxygen therapy (HBOT) has served as an adjunct to excision and grafting in promoting the healing of burn wounds. HBOT has a long history in wound care, especially chronic, complex wounds [13,14]. In 2012, the Undersea and Hyperbaric Medical Society HBOT indications included, among others, acute thermal burn injury [14]. Some burn centers, including our own, use HBOT as a healing adjunct extensively in partial-thickness burns
Clinicians have used ICG-VA and LDI in burn wound depth assessment for over a decade. No report has more objectively and visually documented the effect of HBOT on compromised tissue as recorded by LDI and ICG-VA. In the following case, we used LDI and ICG-VA to characterize a burn wound before and after HBOT prior to surgical management.
Case Report
A 43-year-old female diabetic sustained a burn limited to the plantar surface of her right foot after sleeping in close proximity to a space heater. The ICG-VA used in this case was the SPY Elite system (Lifecell Corp, Branchburg, NJ); the LDI system used in this case was the PeriScan PIM 3 (Perimed AB, Järfälla-Stockholm, Sweden). Five mg of ICG powder was reconstituted with sterile water and injected intravenously rapidly with flush to facilitate ICG-VA. We used both LDI and ICG-VA to characterize her wound at 24 hours post burn (Figure 1). These images reveal particularly poor perfusion to the lateral side of the great toe and the plantar surfaces of the other toes. We started HBOT on this patient, due to her significantly impaired microcirculation secondary to diabetes. The first HBOT treatment was 24 hours after her burn. After two HBOT dives for 90 minutes at 2.0 atmospheres and 100% oxygen, we re-imaged the patient with the LDI and ICG-VA using the same parameters. We performed this imaging approximately 20 hours after her second HBOT dive. There was a definite improvement in clinical appearance and ICG-VA and LDI after two HBOT dives, especially on the medial plantar surface of the great toe, second toe, and inferior portion of the plantar foot burn (Figure 2).
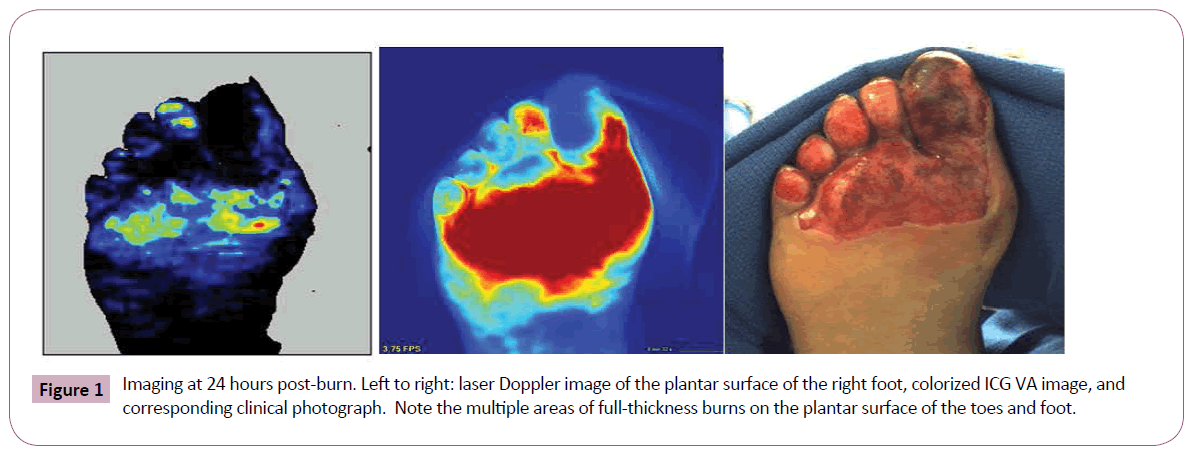
Figure 1: Imaging at 24 hours post-burn. Left to right: laser Doppler image of the plantar surface of the right foot, colorized ICG VA image, and corresponding clinical photograph. Note the multiple areas of full-thickness burns on the plantar surface of the toes and foot.
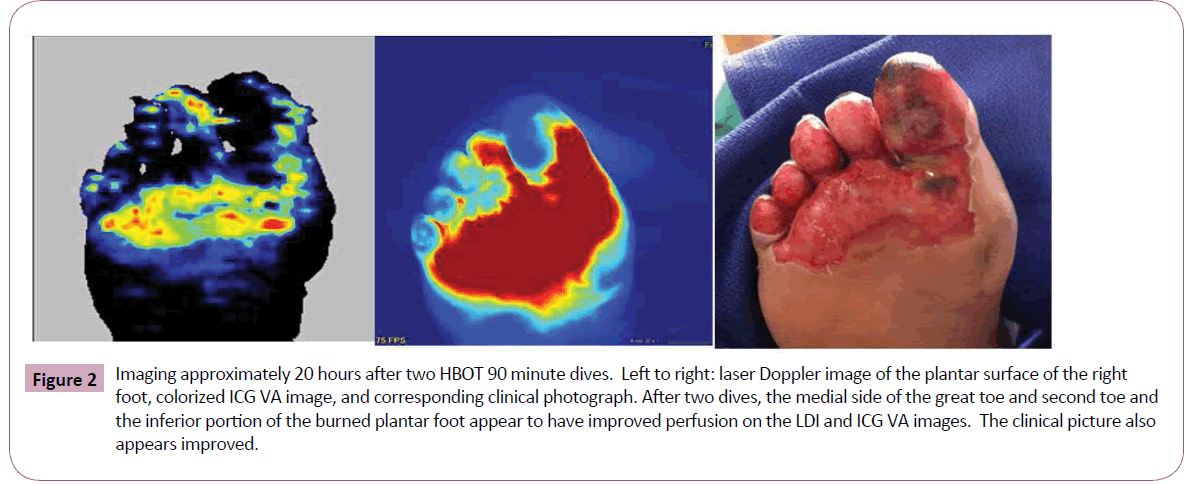
Figure 2: Imaging approximately 20 hours after two HBOT 90 minute dives. Left to right: laser Doppler image of the plantar surface of the right foot, colorized ICG VA image, and corresponding clinical photograph. After two dives, the medial side of the great toe and second toe and the inferior portion of the burned plantar foot appear to have improved perfusion on the LDI and ICG VA images. The clinical picture also appears improved.
We discharged the patient from inpatient care and she subsequently completed three additional dives. No grafting was performed initially on the full thickness toe burns, even though the LDI and ICG-VA predicted no blood flow and no healing. At her two-week follow-up appointment, the plantar surfaces of the toes failed to show any meaningful clinical evidence of healing (Figure 3). We did note improvement in apparent perfusion possibly due to HBOT of several areas-most notably the medial great and second toe and the inferior portion of the plantar foot burn which did not need excision and grafting. At three weeks after her burn, we performed excision and split-thickness skin grafting of the plantar surfaces of her right five toes (Figure 4). Her postoperative course was unremarkable and at subsequent follow-ups we noted successful graft incorporation.

Figure 3: Clinical photograph 16 days after burn. The patient received two HBOT dives while inpatient and three outpatient HBOT dives. The last dive was 10 days before this photograph, which shows worsening clinical appearance, especially of the plantar and lateral surfaces of the five toes.
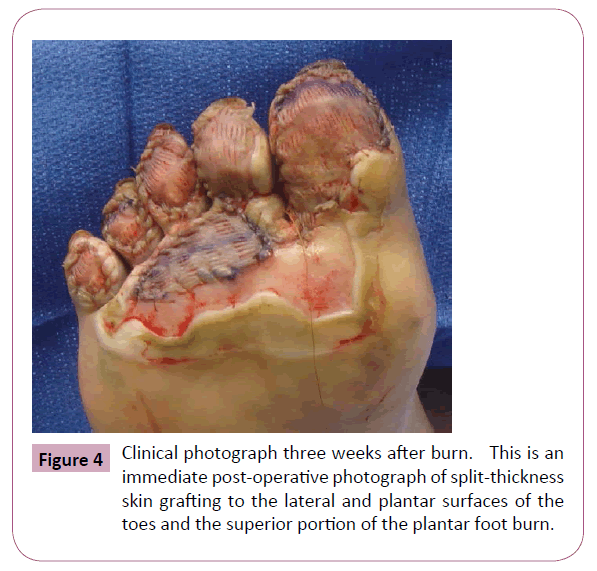
Figure 4: Clinical photograph three weeks after burn. This is an immediate post-operative photograph of split-thickness skin grafting to the lateral and plantar surfaces of the toes and the superior portion of the plantar foot burn.
Discussion
HBOT appears to have a beneficial effect in the healing of burn wounds. While a sizable body of literature supports the use of HBOT in burns, a 2004 Cochrane review failed to establish clear guidelines for the use of HBOT in burn therapy [15]. A 1974 randomized, controlled study demonstrated accelerated healing and lower mortality in acute burn patients who received HBOT [16]. HBOT’s mechanism of providing benefit is controversial, especially at the cellular level. HBOT produces a hyperoxemia which extends to tissue perfusion. The oxygen gradient increases between well-perfused and poorly-perfused tissues, and macrophages respond to these increased oxygen gradients and release angiogenic factors, which have been shown to induce neoangiogenesis in poorly vascularized tissues [17]. Most HBOT protocols involve treatments with 100% oxygen interrupted by brief sessions of room air oxygen to allow physiologic antioxidant mechanisms to prevent overaccumulation of reactive oxygen species (ROS) [16]. The ability for leukocytes to generate ROS in wounds during HBOT is beneficial regarding antimicrobial effects and contributes to prevention of infections [17]. HBOT has few contraindications which include restrictive lung disease and pneumothorax. Complications are extremely rare and include barotrauma and seizures [18].
LDI is already a useful tool in the burn surgeon’s armamentarium. Most studies have evaluated wounds 24-72 hours post burn with LDI [3,8,19] and noted it to be an accurate assessment of depth [8]. LDI is relatively easy to perform, generally takes under 5 minutes per scan, and is completely noninvasive. These advantages have obvious benefit in the management of burn patients, and, as recent research suggests, can significantly decrease cost of treatment and length of stay of burn patients [20]. Even though LDI has no contraindications, the major disadvantages of the LDI systems are their cost and inability to be absolutely calibrated. Furthermore, reliability of LDI is decreased in several situations, including: patient movement, ambient light changes, inability to position the sensor perpendicular to the imaged surface in all situations (i.e. curved surfaces), and application of topical antimicrobials [3].
ICG-VA has some distinct advantages over previously used fluorescent modalities. Fluorescein leaks out of the capillaries into the interstitial tissues where it can remain for up to 12 hours. ICG instead is bound to plasma albumin; it is not nephrotoxic, is rapidly cleared by the liver (plasma half-life of 3-5 minutes), and does not accumulate in the extravascular space and thus can be used multiple times during the same procedure [9,21]. The dose of ICG in ICG-VA manufacturers’ recommendation is a range from 2.5-10 mg, given rapidly intravenously and VA recording begins immediately. Anecdotally, we have our best imaging in burn depth determination after administration of 5 mg of ICG. ICG has a very low side effect profile. The total adverse effect rate of ICG has been reported as 1:42,000; anaphylactic and anaphylactoid reactions, while reported, are extremely rare [9,22]. Because ICG is an iodide, patients with allergies to iodine should not be administered ICG.9 Imaging with ICG-VA is typically recorded in real-time in grayscale, but recently software began to feature the ability to colorize images and place contour lines to more easily delineate between perfused and non-perfused tissue.
In direct comparison, each system has advantages. The ICG-VA, which has real time imaging, is much less sensitive to minor patient movement than LDI, which requires from 1-5 minutes of patient stillness. Both systems enable the user to analyze images after capture. LDI software allows the user to determine the perfusion units in regions of interest. The ICG-VA allows for application of color and contour lines to the original image to more easily delineate perfused and non-perfused areas. Both systems are static snapshots and are limited by the dynamic physiologic parameters which influence tissue perfusion. External factors influencing perfusion are caffeine, alcohol, smoking, bathing, and temporal proximity to the patient’s most recent meal [21]. Moreover, dressings and ointments heavily hamper LDI and ICG-VA ability to evaluate tissue perfusion [21]. Finally, LDI is influenced by hypovolemic shock, which is a considerable factor in acutely injured burn patients [23].
In this case report, we find that LDI and ICG-VA are acceptable methods to objectively document improvement in partial thickness burn wounds after HBOT. Additionally, we suggest that improvement in tissue perfusion with HBOT is likely noted with LDI and ICG-VA more quickly than with clinical observation alone. Of particular note, the LDI and ICG-VA are not very useful in determining tissue perfusion deep to a skin graft immediately after the graft; the clinician must rely exclusively on exam after grafting. Objective measurement of improved perfusion in burn wounds after HBOT is possible with LDI and ICG-VA; both warrant prospective investigation.
Acknowledgment
This research was supported in part by appointment of one of the authors (LCC) to the Knowledge Preservation Program at the U.S. Army Institute of Surgical Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Army Medical Research and Materiel Command.
The opinions or assertions contained herein are the private views of the authors are not to be construed as official or as reflecting the views of the Department of the Army, Department of the Air Force, or the Department of Defense.
8112
References
- Kaiser M, Yafi, A, Cinat M, Choi B, Durkin AJ (2011) Noninvasive assessment of burn wound severity using optical technology: A review of current and future modalities. Burns37: 377-386.
- Jaskille AD, Ramella-Roman JC, Shupp JW, Jordan MH, Jeng JC (2010) Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. J Burn Care Res31: 151-157.
- McGill DJ, Sørensen K, MacKay IR, Taggart I, Watson SB (2007) Assessment of burn depth: a prospective, blinded comparison of laser Doppler imaging and videomicroscopy. Burns 33:833-842.
- Kloppenberg FWH, Beerthuizen GIJM, Ten Duis HJ (2001) Perfusion of burn wounds assessed by Laser Doppler Imaging is related to burn depth and healing time. Burns27: 359-363.
- Jackson DM (1953) The diagnosis of the depth of burning. Br J Surg40: 586-588.
- Godina M, Derganc M, Brcic A (1978) The reliability of clinical assessment of the depth of burns. Burns 4: 92-96.
- Heimbach DM, Afromowitz MA, Engrav LH, Marvin JA, Perry B (1984) Burn depth estimation: man or machine. J Trauma 24:373-378.
- Jeng JC, Bridgeman A, Shivnan L, Thornton PM, Alam H, et al. (2003) Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial. Burns 29: 665-670.
- Brunworth L, Samson M, Newman M, Ramirez J (2011) Nipple-Areola complex evaluation in long pedicled breast reductions with real-time fluorescent videoangiography. PlastReconstr Surg128:585-586.
- Pineda C, Shelton A, Raju N, Welton M (2011) Use of Introperative Fluorescence vascular angiography to assess intestinal perfusion in the creation of intestinal anastomoses. Tech Colo15:215-253.
- Perry D, Bharara M, Armstrong D, Mills J (2012) Intraoperative Fluorescence Vascular Angiography: During Tibial Bypass. J Diabetes SciTechnol6:204-208.
- Skeik N, Porten BR, Isaacson E, Seong J, Klosterman DL, et al. (2014) Hyperbaric Oxygen Treatment Outcome for Different Indications from a Single Center. Ann Vasc Surg S0890-5096.
- Dauwe PB, Pulikkottil BJ, Lavery L, Stuzin JM, Rohrich RJ (2014) Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. PlastReconstr Surg133:208e-215e.
- Villanueva E, Bennett MH, Wasiak J, Lehm JP (2004) Hyperbaric oxygen therapy for thermal burns. Cochrane Database Syst Rev 3:CD004727.
- Hart GB, O’Reilly RR, Broussard ND (1974) Treatment of burns with hyperbaric oxygen. Surg Gynecol Obstet 139:693.
- Marx RE, Stern D(2012) Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Quintessence: Chicago 390-391.
- Ray CS, Green G, Cianci P (1991) Hyperbaric oxygen therapy in burn patients: cost effective adjuvant therapy. Undersea Biomed Res 18:77.
- Pape SA, Skouras CA, Byrne PO (2001) An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 27:233-239.
- Venclauskiene A, Basevicius A, Zacharevskij E, Vaicekauskas V, Rimdeika R, et al. (2014) Laser Doppler imaging as a tool in the burn wound treatment protocol. WideochirInne Tech MaloInwazyjne. 9:24-30.
- Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, et al. (2013) Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 7:1.
- Benya R, Quintana J, Brundage B (1989) Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn17:231-233.
- Cancio LC, Batchinsky AI, Mansfield JR (2006) Hyperspectral imaging: a new approach to the diagnosis of hemorrhagic shock. J Trauma60:1087-1095.










