Lipinski B*
Joslin Diabetes Center, Harvard Medical School, One Joslin Pl, Boston MA 02215, USA
- *Corresponding Author:
- Lipinski B
Joslin Diabetes Center, Harvard Medical School, One Joslin Pl, Boston MA 02215, USA
Tel: 617-953-5202
Fax: 617-309-2667
E-mail: b.lipinski2006@rcn.com
Received April 25, 2016; Accepted May 18, 2016; Published May 23, 2016
Citation: Lipinski B (2016) On the Role of Insoluble Fibrin Clots in Tumor Stroma. Int J Drug Dev & Res 8: 028
It was suggested some years ago that solid tumors escape immunological recognition and destruction by coating themselves with a layer of insoluble fibrin [1]. However, very little data was available at that time to support this notion. Only very recently and important paper was published describing insoluble fibrin deposits in the stroma of various tumors that are different from soluble fibrinogen [2]. The authors explain this finding in terms of the malignant cycle of blood coagulation, in which tissue factor is suggested to play an important role in tumor proliferation, invasion and metastasis. They have also indicated that in non-malignant diseases fibrin is gradually removed due to the plasmin fibrinolytic activity, yet in malignant tumors fibrin deposits persist as long as cancer cells survive in the body.
It should however, be noted that here are other pathological instances, in which fibrin is not readily eliminated from the human organs, for example in stroke patients. It is known that cerebral thrombi can be resolved by means a thrombolytic therapy only when installed within 4-5 hours after the onset of thrombosis, but become refractory to fibrinolysis at later times. This was explained in terms of the free radical-induced structural modification of fibrin that makes it resistant to fibrinolysis [3]. Subsequently, it was reported that such a modification can be initiated by the exposure of blood to the redoxactive iron (Fe+3) that accumulates in the human body as a result of excessive consumption of red meat and/or red cell hemolysis induced by environmental toxins. The proposed mechanism of fibrinogen modification involves a hydroxyl radical-induced reduction of intramolecular disulfide bridges in the fibrinogen molecule. This results in the exposure of buried hydrophobic epitopes followed by the formation of inter-molecular bonds resistant to the action of proteases [4]. Of note, similar hydrophobic modifications of fibrinogen can be initiated by the exposure of human blood to the action of a specific dithiol reducing agent [5]. This particular reaction is effectively inhibited by redox-active sodium selenite that can also abolish Ebola virus infectivity by virtue of the inhibition of the viral protein disulfide isomerase [6]. In addition, sodium selenite can directly neutralize redox iron by converting it to the inactive divalent ferrous ion (Fe+2).
It should also be mentioned that certain cancers were shown to accumulate insoluble fibrinogen without the action of thrombin [7,8]. This important, albeit at that time not fully understood phenomenon, can now be explained in terms the iron-induced pathway of blood coagulation [4] leading to the deposition of insoluble fibrin (parafibrin) in the tumor stroma. The most important feature of parafibrin is its total resistance to the proteolytic degradation thus making it refractory to the cellular immune destruction. However, the protective coat of parafibrin can be removed from the tumor cell surface by means of the action of certain of certain natural hydrophilic substances notably polyphenols (Figure 1). Beneficial health effects of polyphenolic compounds, including their anticancer properties, have been well recognized [9,10]. In conclusion, I argue that the hydrophobic interactions on the surface of cancer cell membranes may become a target for a novel class of anticancer drugs.
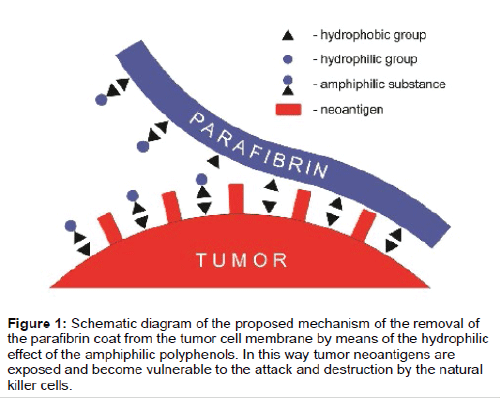
Figure 1: Schematic diagram of the proposed mechanism of the removal of the parafibrin coat from the tumor cell membrane by means of the hydrophilic effect of the amphiphilic polyphenols. In this way tumor neoantigens are exposed and become vulnerable to the attack and destruction by the natural killer cells.
9487
References
- Lipinski B, Egyud LG (2000) Resistance of cancer cells to immune recognition and killing. Med Hypotheses 54: 456-460.
- Obonai T, Fuchigami H, Furuya F, Kozuka N, Yasunaga M, et al. (2016) Tumour imaging by the detection of fibrin clots in tumour stroma using an anti-fibrin Fab fragment. Sci Rep 6: 23613.
- Lipinski B (2010) Modification of fibrin structure as a possible cause of thrombolytic resistance. J Thromb Thrombolysis 29: 296-298.
- Lipinski B, Pretorius E (2012) Novel pathway of iron induced blood coagulation: implications for diabetes mellitus and its complications. Pol Arch Med Wewn 122: 115-122.
- Lipinski B, Egyud LG (1992) Thiol-induced crosslinking of human blood proteins: Implications for tumor immunity. Bioorg Med Chem Lett 2: 919-924.
- Lipinski B (2014) Can selenite be an ultimate inhibitor of Ebola and other viral infections? Br J Med Medic Res 6: 319-324.
- Costantini V, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, et al. (1991) Fibrinogen deposition without thrombin generation in primary human breast cancer tissue. Cancer Res 51: 349-353.
- Copley AL (1981) Hemorheological aspects of the endothelial fibrin lining and fibrinogen gel clotting. Their importance in physiology and pathological conditions. Clin Hemorh 1: 9-72.
- Lipinski B (2011) Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 2011: 809696.
- Maru GB, Hudlikar RR, Kumar G, Gandhi K, Mahimkar MB (2016) Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J Biol Chem 7: 88-99.







