Keywords
Optical coherence tomography; Multiple sclerosis; Optic neuritis; Automated perimetry; VEP
Introduction
Optical coherence tomography is a non-contact and non-invasive method for examination of retinal layers by the measurement of their thickness that also allows for precise qualitative follow-up of the development of pathologic processes in these layers. This technique was introduced in 1991 by Huang et al. [1] and since then its applicability for examination of visual functions of patients with MS has been studied in multiple trials that included patients with acute ON, with ON in the past and patients who have never had ON. These studies demonstrated the value of OCT for the evaluation of changes caused by the demyelination of the optic nerve and visual pathways, as well as for the monitoring of the recovery of their functions. allows for examination of structures made up entirely of axons (the non-myelinated axons in the structure of the RNFL) [2], as well as ganglion cells in the retina (GCC) in the macular area. Many studies demonstrated thinning of RNFL and GCC (as more closely correlating with the atrophic changes in MS patients) after ON [3-7]. A lot of studies have also been performed to compare the thickness of the layers in MS patients who have never had ON with those in healthy people [2,8-12]. Vision-related symptoms may be present in patients suffering from MS who have normal visual acuity and in those who don't have history for an episode of acute ON in the course of their disease [13-15]. examinations show more advanced changes in patients with ON in the past and most authors find that atrophic changes can be established by OCT approximately 3 months after the acute ON. Costello et al. presented data from longitudinal studies of RNFL thickness over 12 months after acute ON, showing that axonal loss in the eye continues for at least 1 year and the most substantial decrease takes place approximately 6 months after the acute episode [16]. A metaanalysis by Petzold et al. [17] of data from more than 30 OCT studies in MS patients shows decrease of the RNFL thickness by 20.38 μm in MS patients after ON and by 7.08 μm in patients who never had ON and that this thinning is more severe than the anticipated decrease caused by normal ageing in healthy people and is probably due to trans-synaptic retrograde degeneration in the absence of ON. In our study we have compared the findings for these retinal layers in three groups of MS patients and in healthy volunteers.
Purpose
The purpose of our study was to compare the thicknesses of RNFL and GCC layers, affected by the processes of demyelination and neurodegeneration, in three groups of MS patients - with acute ON, with ON in the past (more than 6 months ago) and with no history for ON, and in a control healthy group. In addition, we aimed to collate the results from OCT with the findings from functional measurements for visual disorders in MS - automated perimetry (AP) and visual evoked potentials (VEP) of the patients to find if there was significant correlation between these techniques.
Materials
We examined a total of 202 eyes, 31 of them with acute ON at the moment of examination, 50 with ON more than 6 months ago and 121 eyes with no evidence for optic neuritis, of 101 patients with proved multiple sclerosis as per the latest diagnostic criteria. All patients examined by us regarding their visual status, have been diagnostically confirmed as suffering from clinically definite MS according to the 2010 Revised McDonald Diagnostic Criteria for MS. The separation in groups was made on the basis of the status regarding history for optic neuritis of each eye, due to the fact that there are many cases of patients with different status for each of their eyes, e.g. one eye with acute ON and the other eye with ON in the past or without ON. Therefore it is not possible to assign the patients to one of the groups as a whole, but the morphological and functional examinations and the statistical analyses, respectively, were performed for each eye separately. 64 of the patients (64.64%) are female and 37 (37.36%) are male. The mean age of the patients is 36.50 years (± 10.13), with minimal age of 10 years and maximal age of 61.
The results of the patients were compared with those of 94 eyes of 47 healthy volunteers, selected from a healthy population with no system diseases or ophthalmologic pathologies that could be associated with visual disorders, with visual acuity 1.0 and no refraction abnormalities exceeding ± 2 Dpt. The mean age of control subjects is 36.25 years (± 11.9), with minimal age of 16 years and maximal age of 60, and the sex distribution is 27 females (57.45%) and 20 males (42.55%).
Methods
We performed a comprehensive set of neuroophthalmological examinations for all patients: determination of the best corrected visual acuity, assessment of the low contrast sensitivity (with Pelli-Robson test), examination of the color vision with Ishihara tables, check of eye movements and fundus examination. Most of the patients had VEP testing (with determination of the latency at P100 and the amplitudes of the components N75-P100 and P100-N145) and visual field testing using automated perimetry. examination was carried out in all patients (FD-OCT with a RTVue-100 unit, software version 6.11.1.12, Optovue), using programs for thickness determination for the retinal nerve fiber layer (RNFL) and the macular ganglion cell complex (GCC).
Results and Conclusion
Optic neuritis (ON) is an inflammation of the optic nerve caused by various etiologic factors. Its incidence in MS patients demyelination process. Based on the data of Leibowitz et al. up to 50% of the patients suffering from MS have a decrease is very high due to involvement of the nerve by the or loss of vision as their onset MS symptom and up to 80% experience visual disorders at some stage of the disease [18,19]. The neuro-ophthalmological findings in our patients were as follows (by groups).
Eyes with acute optic neuritis
We examined 30 MS patients with acute ON (29 with unilateral acute ON (96.66%) and 1 patient with bilateral acute ON (3.33%) (i.e. 31 examined eyes). In almost all patients the onset of the condition was acute/subacute with development of visual decrease and pain in the affected eye within several hours or up to 1 day. In 14 (46.6%) of the patients this was the first manifestation and the reason for diagnosis of MS, and in 16 (53.33%) the acute ON was a new episode of previously diagnosed MS. The clinical profile of eyes with acute ON episode included: decreased visual acuity of various degrees of severity, from complete lack of perception to 1.0, e.g. 12 (38.7%) of the patients had visual acuity of 0.01 to 0.2, while 3 (9.67%) of them had visual acuity 1.0; presence of relative afferent pupillary defect (RAPD) on slit-lamp examination in 19 (61.29%) of the eyes; various colour vision abnormalities - complete lack of colour vision at the moment of examination in 10 eyes (32.26%) with acute ON and different degrees of pathology in the rest of the eyes to normal perception of colours; decreased contrast sensitivity tested by the Pelli- Robson test in almost all eyes affected by acute ON, with complete lack of perception for low contrast symbols in 9 eyes (29.03%) - the eyes with very low visual acuity, and various degrees of severity between 1.35 and 1.95 for the rest of the eyes; visual field defects with automated perimetry are present in 22 (70.97%) of the eyes with acute ON with central scotoma as the most frequent defect - in 10 eyes (32.25%), 1- quadrant defect in 5 eyes (16.13%), 2-quadrant defect in 2 eyes (6.45%), paracentral scotoma in 2 eyes (6.45%), and 3- or 4-quadrant defects in individual cases; VEP abnormalities with prolonged latency 100 (in 88.89%) and decreased amplitude for the N75-P100 and P100-N145 components (in 100% of the eyes with acute ON). The statistical analyses showed statistically significant impact of the group factor on the three evaluated VEP parameters. examination demonstrated significant decrease in RNFL and GCC thickness in the affected eyes in comparison with the unaffected eyes of the patients. The average RNFL thickness in the eyes with acute ON was 87.16 μm (± 17.032), the min. measured value was 54 μm and the max. value - 117 μm. For the thickness of the ganglion cell complex (GCC) the average value was 86.08 μm (± 13.118), the min. measured value was 59 μm and the max. one - 116 μm. There is also a significant difference when compared with the parameters in the examined healthy volunteers. The average thickness in the control group was 110.28 μm for RNFL and 99.82 μm for GCC.
Eyes with ON in the past
The neuro-ophthalmological findings in the eyes with ON in the past (more than 6 months ago) examined by us (n=50) included: the visual acuity in most of the cases is recovered to various degree after the acute episode, with residual visual decrease in part of them and normal visual acuity in other cases (in 38% of our patients); absence of RAPD on slit-lamp examination; temporal or diffuse papillary pallor resulting from the partial or complete atrophy of the optic nerve is found with fundus examination; various degrees of colour vision abnormalities - complete recovery of the colour vision with 14/14 score by Ishihara tests in 29 eyes (58%) with ON in the past, and residual disorder of various severity in the rest of the cases; contrast sensitivity evaluated by the Pelli-Robson test also demonstrates recovery in most of the cases, but in 9 eyes (18%) it is decreased to 1.65, while in 9 other eyes it is decreased to 1.35; visual field abnormalities with automated perimetry are present in 16 (32%) of the eyes with ON in the past, most frequently diffusely decreased sensitivity to light - in 10 eyes (20%), central scotoma - in 2 eyes (4%), 1-quadrant defect in 2 eyes (4%) and concentric narrowing of the visual field in 2 eyes (4%). There is complete recovery of the visual field of the affected eye in the rest of the cases; VEP abnormalities with prolonged latency 100 (in 86.67%) and decreased amplitudes - for the N75-P100 component (in 100% of the cases) and the P100-N145 component (in 93.10%). OCT demonstrated severe decrease of RNFL and GCC thickness in the examined eyes with ON in the past in comparison with the unaffected eyes that never had ON. The average RNFL thickness in the eye with ON in the past of the patients was 83.38 μm (± 11.317), the min. measured value was 57 μm and the max. - 111 μm. GCC thickness values are: mean value of 79.18 μm (± 7.457), min. - 67 μm and max. - 97 μm, respectively. In comparison with the eyes with acute ON, the eyes with ON in the past (more than 6 months ago) show more severe changes, probably due to the time needed for the development of atrophic changes. With follow-up of the patients with acute ON, the measured parameters already showed more severe degenerative changes several months later.
Eyes with no history for ON
The findings in MS patients with no history for ON (n=121) are as follows: normal visual acuity, there is mild decrease (visual acuity 0.7-0.8) in only 4 of the examined eyes (3.3%), while in the remaining 117 eyes (96.69%) it is 1.0; presence of temporal or diffuse papillary pallor is occasionally found on fundus examination despite of the lack of history for ON, as manifestation of subclinical involvement of the visual pathways by the neuro-degeneration process; normal colour vision; normal contrast sensitivity on examination with the Pelli-Robson test - there is decreased contrast sensitivity of up to 1.65 on monocular testing with normal result on binocular testing, only in 5 eyes (4.13%); normal visual field with automated perimetry; VEP changes with prolonged latency 100 (in 84.78% of the cases) and decreased amplitude for N75-P100 component (in 89.01%) and P100-N145 component (in 91.01%). OCT examination of our patients without ON demonstrated decrease in RNFL and GCC thickness in comparison with healthy volunteers. The average thickness of RNFL in the eyes without ON of the examined patients was 99.13 μm (± 14.482), with minimal measured value of 66 μm and maximal value of 147 μm. The respective average thickness value for GCC is 92.25 μm (± 13.609). In the control group the average RNFL thickness is 110.28 μm, and the average GCC thickness is 99.82 μm. The results are summarized in Table 1.
| |
Age distribution |
Sex distribution |
Area of living |
Occupation |
| |
< 18 years |
18-30 years (incl.) |
30-50 years (incl.) |
>50 years |
Female |
Male |
Urban |
Rural |
White collar |
Blue collar |
| Acute ON (n=30) |
2 |
14 |
13 |
1 |
17 |
13 |
20 |
10 |
25 |
5 |
| ON in the past (n=32) |
|
5 |
22 |
5 |
24 |
8 |
23 |
9 |
24 |
8 |
| No ON (n=39) |
|
7 |
27 |
5 |
23 |
16 |
29 |
10 |
30 |
9 |
| MS patients, total (n=101) |
2 |
26 |
62 |
11 |
64 |
37 |
72 |
29 |
79 |
22 |
Healthy volunteers
(n=47) |
2 |
12 |
27 |
6 |
27 |
20 |
39 |
8 |
41 |
6 |
| Total |
4 |
38 |
89 |
17 |
91 |
57 |
111 |
37 |
120 |
28 |
Table 1: Demographics of MS patients and healthy volunteers.
In comparison with the parameters in patients with ON in the past (more than 6 months ago) and acute ON, the changes in the eyes with no history for ON are quite milder, but statistically significant. RNFL and GCC thickness correlates with the data for subclinical changes from the functional electrophysiological VEP testing which shows asymmetry and prolonged latency, as well as the frequently found pallor of the optic nerve papilla with fundus examination, and support the presence of "occult vision loss", described by many authors [14,20,21] in MS patients as a sign of the general neurodegeneration process in the body. Recent studies show that one of the most sensitive methods for detection of visual functions disorder in these patients is contrast sensitivity examination with the Pelli-Robson test [22] which also correlates well with VEP examination [23]. This test can be performed by either binocullar examination to test the overall vision or for each eye separately to assess the function of each optic nerve [24,25].
Our findings for the eyes with ON in the past do not show complete correlation with the clinical symptoms which are recovered to a large extent in comparison with the ones during the acute episode of ON (with normal or almost recovered visual acuity, recovered visual field defects, normalized colour vision and improved VEP parameters), while the morphological OCT parameters demonstrate serious degeneration of the examined layers. Findings in the eyes without ON in these patients do not correlate with the clinical parameters as well (normal visual acuity, colour vision and perimetry), because OCT and VEP show the presence of subclinical visual disorders with the background of otherwise normal visual functions (Figure 1).
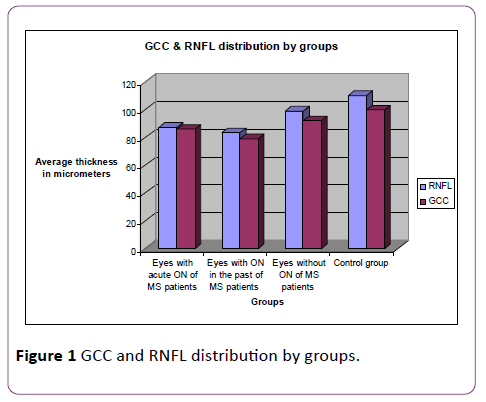
Figure 1: GCC and RNFL distribution by groups.
Statistical analysis of our data demonstrated the following findings: The descriptive statistical methods showed that the most severe decrease of RNFL thickness is observed in the group with ON in the past (more than 6 months ago), it is less severe in the eyes examined during the acute episode of ON and the mildest (and yet statistically significant) decrease is found in the eyes that have never been affected by optic neuritis, in MS patients in comparison with the eyes of healthy control subjects. The single factor analysis of variance (ANOVA) of RNFL data showed statistically significant influence of the group factor on RNFL values, F=54.434, p<0.0001. ANOVA analysis was further completed by post hoc tests to check which groups show intergroup differences in the mean values. The post-hoc tests for multiple comparison demonstrated the presence of statistically significant difference between the mean values of eyes with ON in the past and eyes with no history for on one hand, and between them and eyes with acute ON, on the other hand. There was statistically significant difference with the eyes of healthy controls as well (p=0.001).
For the other examined parameter, GCC, we also found the most severe thickness decrease in patients with ON more than 6 months ago, less severe decrease in eyes with acute ON and the mildest (but yet statistically significant) thickness decrease in eyes that have never been affected by ON. The analysis of variance for GCC data shows that there is statistically significant influence of the group factor on GCC thickness, F=44.008, p<0.0001. The post-hoc tests for multiple comparison showed statistically significant difference between the mean values of eyes with ON in the past and eyes with no history for ON, as well as difference with the eyes of the volunteers (p<0.005).
We looked for correlations between all examined parameters through parametric methods for all three groups together and for each of them separately. For the first group, consisting of eyes with acute ON at the time of examination, we found correlation between RNFL and GCC (R=0.398, p<0.05). For the second group (eyes with ON in the past) we found statistically significant correlation between RNFL and GCC (parameters with normal data distribution) (R=0.679, p<0.01). In the group without ON there was statistically significant relationship between RNFL and GCC parameters (R=0.413, p<0.01) as well. Upon parametric correlation analysis for all three groups of MS patients together, we found correlation between RNFL and GCC (R=0.538, p<0.01).
and visual evoked potentials
For VEP findings we used non-parametric methods for statistical analysis of the parameters VEP latency ( 100) and amplitude N75-P100, and parametric methods for analysis of the amplitude P100-N145. The one-factor analysis of variance showed presence of statistically significant influence of the group factor on VEP amplitude P100-N145 (F=4.816, p<0.05). We applied Kruskal-Wallis rank test for analysis of the results for VEP latency (100) and amplitude N75-P100 and demonstrated the presence of statistically significant influence of the group factor on VEP latency as well as on VEP amplitude N75-P100 (0.016, p<0.05).
When comparing results from VEP examinations and OCT findings using parametric methods for correlation analysis for the eyes in the group with ON in the past we established statistically significant correlation between the parameters RNFL and VEP amplitude P100-N145 (R=0.504, p<0.01).
When using non-parametric methods to find a correlation between RNFL and the parameters without normal data distribution (VEP latency (100) and VEP amplitude N75-P100), we found statistically significant correlation between RNFL and VEP latency (-0.342, p<0.01) and between RNFL and VEP amplitude N75- 100 (0.266, p<0.05) for the group of eyes with no history for ON. When applying non-parametric correlation analysis for all three groups of MS patients together, we found presence of correlation both between RNFL and VEP latency (0.380, p<0.01), and between RNFL and VEP amplitude N75-100 (0.298, p<0.01).
Apart of this we looked for correlations between GCC and VEP amplitude (P100-N145) and found statistically significant association both in the group of eyes with acute ON (R=0.552, p<0.05), the group with ON in the past (R=0.473, p<0.01), and the group of eyes without ON (R=0.267, p<0.05), as well as for all three groups together (R=0.340, p<0.01). When using nonparametric methods to search correlations between GCC and the parameters without normal data distribution (VEP latency ( 100) and amplitude (N75-P100)), we found statistically significant correlation between GCC and VEP amplitude N75- 100 (0.587, p<0.015) for the eyes with acute ON, and correlation between GCC and VEP amplitude N75- 100 (0.375, p<0.05) in the group with ON in the past. In the group of eyes without history for ON we demonstrated statistically significant correlations both between GCC and VEP latency (100) (0.419, p<0.01), and between GCC and VEP amplitude N75- 100 (0.219, p<0.05). When using nonparametric correlation analysis for all three groups of MS patients together, we found correlations both between GCC and VEP latency (0.503, p<0.01) and between GCC and VEP amplitude N75- 100 (0.362, p<0.01).
The findings described by us correspond to the data from OCT studies in MS patients reported in the literature thus far that have demonstrated a decrease in RNFL thickness in the range 5-40 micrometers, with an average of 20.38 μm (summarized data from studies in MS patients during the last years [3,13,15].
Conclusion
In conclusion, we demonstrated the high value of as a method for precise assessment of the changes in morphological indicators for retinal damage in MS patients with acute optic neuritis, after ON and without history for ON and provided data on statistically significant correlations between OCT and other routinely used methods for functional examination of visual functions like the visual evoked potentials (Table 2).
| |
Eyes with acute ON of MS patients,
average (n=31) |
Eyes with ON in the past of MS patients,
average (n=50) |
Eyes without ON of MS patients,
average
(n=121) |
Control group (n=94) |
| RNFL |
87.16 μm
(± 17.032 μm) |
83.38 μm
(± 11.32 μm) |
99.13 μm
(± 14.482 μm) |
110.28 μm
(± 10.769 μm) |
| GCC |
86.08 μm
(± 13.118 μm) |
79.18 μm
(± 7.457 μm) |
92.25 μm
(± 13.609 μm) |
99.82 μm
(± 5.846 μm) |
Table 2: Comparison of RNFL and GCC thickness between eyes with acute ON, ON in the past and without history for ON, and the healthy control group.
Acknowledgements
OCT examination of the patients was financed by the Medical University - Sofia by "Young investigator" 8-D/2012 project.
18002
References
- Albrecht P, Froehlich R, Hartung H (2007) Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol 254:1595-1596.
- Baier M, Cutter G, Rudick R (2005) Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology 64:992-995.
- Balcer L (2004) Multiple sclerosis and related demyelinating diseases. In: Miller N., Newman N, Biousse V, Kerrison J, eds. Walsh and Hoyt's Clinical Neuro-ophthalmology. Vol. 3 (6thedn). Philadelphia: Lippincott Williams & Wilkins 2004:3429-3525.
- Bock M, Brandt A, Doerr J (2010) Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. ClinNeurolNeurosurg 112: 647-652.
- Burkholder B, Osborne B, Loguidice M (2009) Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol66:1366-1372.
- Costello F, Hodge W, Pan Y (2008) Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Multiple sclerosis 14:893-905.
- Fjeldstad C, Bemben M, Pardo G (2011) Reduced retinal nerve fiber layer and macular thickness in patients with multiple sclerosis with no history of optic neuritis identified by the use of spectral domain high-definition optical coherence tomography. J ClinNeurosci 18: 1469-1472.
- Fisher J, Jacobs D, Markowitz C (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Opthalmology 113:324-232.
- Huang D, Swanson E, Lin C (1991) Optical coherence tomography. Science 254:1178-1181.
- Jeanjean L, Castelnovo G, Carlander B (2008) Retinal atrophy using optical coherence tomography (OCT) in 15 patients with multiple sclerosis and comparison with healthy subjects. Rev Neurol164:927-934.
- Klistorner A, Arvind H, Nguyen T (2008) Axonal loss and myelin in early on loss in postacute optic neuritis. Ann Neurol 64:325-331.
- Kupersmith M, Nelson J, Seiple W (1983) The 20/20 eye in multiple sclerosis. Neurology 33:1015-1020.
- Leibowitz U, Alter M (1968) Optic nerve involvement and diplopia as initial manifestations of multiple sclerosis. ActaNeurolScand 44:70-80.
- Leys MJ, Candaele CM, De Rouck AF, Odom JV (1991) Detection of hidden visual loss in multiple sclerosis. A comparison of pattern-reversal visual evoked potentials and contrast sensitivity. Doc Ophthalmol 77: 255-264.
- Newman N, Wolfe J, Stewart M (1991) Binocular visual function in patients with a history of monocular optic neuritis. Clin Vis Sci6:95-107.
- Parisi V, Manni G, Spadaro M (1999) Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 40:2520-2527.
- Petzold A, Boer J, Schippling S (2010) Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol 9: 921-932.
- Pueyo V, Ara J, Almarcegui C (2010) Sub-clinical atrophy of the retinal nerve fiber layer in multiple sclerosis. ActaOphthalmol 88:748-752.
- Regan D, Silver R, Murray T (1977) Visual acuity and contrast sensitivity in multiple sclerosis - hidden visual loss: An auxiliary diagnostic test. Brain 100:563-579.
- Rubin G, Munoz B, BandeenRK (2000) SEE Project Team. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Invest Ophthalmol Vis Sci 41:3327-3334.
- Saidha S, Syc S, Durbin M (2011) Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. MultScler17:1449-1463.
- Sisto D, Trojano M, Vetrugno M (2005) Subclinical visual involvement in multiple sclerosis: a study by MRI, VEPs, frequency-doubling perimetry, standard perimetry and contrast sensitivity. Invest Ophthalmol Vis Sci 46:1264-1268.
- Sorensen T, Frederiksen J, BronnumHH (1999) Optic neuritis as onset manifestation of multiple sclerosis: A nationwide, longterm survey. Neurology 53:473-478.
- Trip S, Schlottmann P, Jones S (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58:383-391.
- Walter S, Ishikawa H, Galetta K (2012) Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology119:1250-1257.






