Keywords
Ozonated oil, Staphylococcus aureus, methicillin resistant Staphylococcus aureus
Introduction
Disruption of normal skin and depression of immune-system are the major risk factors for exogenous infection in burn patients. Among several of hospital associated infections, gram positive bacteria “Meticillin Resistance Staphylococus aurious (MRSA)” is one of the common bacteria that cause serious problems in burns. Clinical importance of MRSA in burns highlighted by several groups and appropriate therapeutic measures has been attempted to minimize the transmission and infection in vulnerable group of patients [1]. Microorganisms transmitted from the hospital environment tend to be more resistant to antimicrobial agents than those originating from the patient's normal flora [2, 3]. Ozone is well recognized as antibacterial agent and therapeutic effect of ozonated compound or oil have been reported and validated by several cutaneous wound healing which emphasizes the importance of ozonated oil [4-9]. However for burns, the effect of ozonated oil in the treatment of MRSA has not been reported by to date. Hence this study aimed to evaluate the microbicidal effect of ozonated oil and its efficacy in regression of burn infected MRSA growth.
Methodology
Sample Collection
The current study was conducted at National Burn Centre, by taking burn wound swabs of burn patients during the period of six month. All the wound swab samples were collected by using convenient sampling technique from all “in-patients” and “out-patients” during the study period. The wound swabs were collected by the attending physician or health officer using sterile applicator stick with cotton swabs moistened with normal saline and test tubes. The sample collection, culturing, staining and sensitivity tests were performed according to National Burns Centre guide lines for standard microbiological diagnosis. The patient details such as age, sex and socio-demographic details were taken from patient’s card.
Sterilization
Maintaining sterile conditions is essential when working with biological samples. All work involving the use of biological samples was conducted in Class II safety cabinets. In each step of procedure 70% alcohol solutions or bleach were used to disinfect the surfaces and any containers entering or leaving the cabinet. Sterilization of media and any equipment used in cabinets was conducted by autoclave, which uses heat sterilization method. The autoclave system decontaminates solutions and equipment at 121°C and 15psi for 15mins.
Identification of the bacteria
A small portion of the sample is swabbed onto a glass slide. This is then stained with Gram stain or dyes like crystal violet and basic fuschin and viewed under the microscope. S. aureus is Gram positive and stains blue or purple and appears as small round cocci or short chains and most commonly as grape-like clusters. Since S. aureus may be normally present on skin and mucous membranes, this test is not always confirmatory.
Colony detection by Tissue Culture plate method
The wound swab specimens were stricken to the blood agar plate. The micro biota was detected by tissue culture plate method (TCP). The swabs were then inoculated on blood agar plate and incubated at 37ºc for 24 hrs. After 24 hrs the plates were brought to room temperature and isolated colonies were selected. If scarcity of isolated colony, then sub-cultures were done to get isolated colony. After 24 hours, single colonies were taken in liquid suspension and gram staining was done.
Identification of MRSA by biomerieux vitek2 Compact system
Streak plate technique was used for the isolation of pure culture of the S. aureus, from mixed population of swab collection. The inoculums were streaked over the agar surface in such a way that it “thins out” the bacteria. Some individual bacterial cells were separated and well-spaced from each other. As the original sample was diluted by streaking over successive quadrants, the number of organism’s decreases and colonies were formed. Usually by the third or fourth quadrant only a few organisms were transferred which gave discrete colony forming units (CFUs). The colony was picked up from quadrant 4 and gram staining was done to ensure the gram positive bacteria. Based upon the gram positive was chosen, hence Gram positive VITEK 2 Compact card (GPC) card or GP card was used. Suspension culture was made by picking up colony from the fourth quadrant. Machine work was done by putting cassette in filler section followed by pressing start fill button. Then the steps were followed according to command of machine. All the software work was carried out according to the manufactures protocol. The result was analyzed based upon the reports received. By using VITEK 2 GP COLORIMETRIC Identification Card, MRSA positive was screened by specific box corresponding to the individual strain and antibiotic name [10] .
Coagulase Test
A staphylococcal colony was emulsified in a drop of water (30 to 40 microliter) on a clean and grease free glass slide with a minimum of spreading. Similar suspensions of control positive and negative strains were made to confirm the proper reactivity of the plasma. A flamed and cooled straight inoculating loop was dipped into the undiluted plasma at room temperature, it was withdrawn, and the adhering traces of plasma (not a loopful) were stirred into the staphylococcal suspension on the slide. The loop was flamed and repeated for the control suspensions. A coarse clumping of cocci visible to the naked eye within 10 seconds was read as positive. The absence of clumping or any reaction taking more than 10 seconds to develop was read as negative, but were re-examined for any slow reacting strains by the tube coagulase test in which the free coagulase enzyme forming clumps.
Growth Curve analysis
1ml LB broth culture was transferred to a 5 ml tube to be used as a blank later. The remaining LB broth was inoculated with 5ml overnight culture. The flask was gently swirled in a circular motion to thoroughly mix. After transferring 1ml of culture to a cuvette, OD was taken in every 2 hours using spectrophotometer at 580 nm setting the blank at zero. Growth curve analysis was done for 10 hours where time represented in x-axis and absorbents in y-axis. The curve showed three phases of bacterial growth – lag, log and stationary as shown in Figure 1.
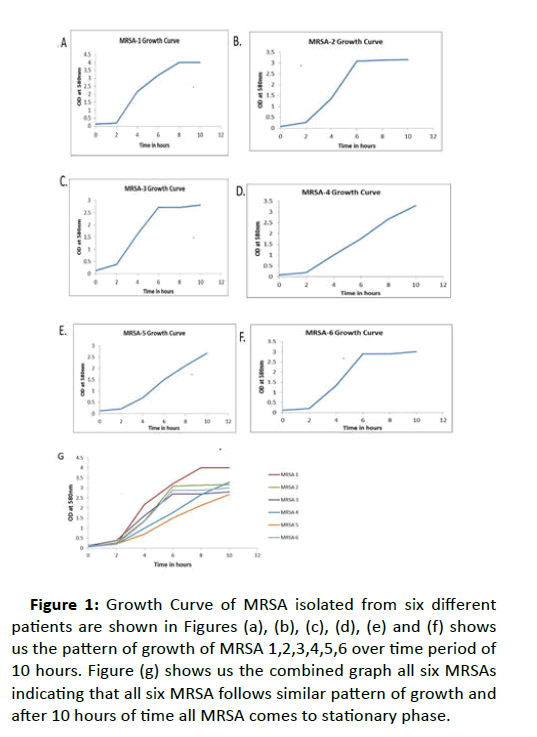
Figure 1:Growth Curve of MRSA isolated from six different patients are shown in Figures (a), (b), (c), (d), (e) and (f) shows us the pattern of growth of MRSA 1,2,3,4,5,6 over time period of 10 hours. Figure (g) shows us the combined graph all six MRSAs indicating that all six MRSA follows similar pattern of growth and after 10 hours of time all MRSA comes to stationary phase.
Preparation of dilutions for plating and susceptibility test
Four natural components such as (i) Shata Dhauta Ghruta, (ii) Jatyadi Oil, (iii) Ozonated Oil, (iv) Ozonated Oil were selected. Mueller-Hinton Agar media was made mixing MH Agar in distilled water and autoclaving it to digest the agar and sterilize it. Different concentrations of the components were prepared with the help of the tables given below for each component (Tables 1-4). For susceptibility Test by “Disc Method”, a pure culture plate of MRSA was selected. A sterile Mueller-Hinton agar (MHA) plate was taken. The swab with the test organism (MRSA) was used to streak a MHA plate. After the streaking is complete, the plate was allowed to dry for 5 minutes. Discs dipped in novel components were placed on the surface of the agar using sterilized forceps. The inoculated plates were then carefully inverted and incubated for 24 hours at 37° C. After incubation, the plates having growth of microorganisms was observed and checked for a zone of inhibition [11]. For “Spread Plate Method”, a pure culture plate of MRSA was selected. A sterile Mueller-Hinton agar (MHA) plate was taken with the dilutions mixed in it. The swab with the test organism was used to streak a MHA plate. The inoculated plates were then carefully inverted and incubated for 24 hours at 37° C. After incubation, the plates were checked for growth.
| Sl. No |
Microorganism infection |
Number of patients infected |
% of infection |
| 1 |
Pseudomonas aeruginosa |
152 |
58 |
| 2 |
Acinetobacter baumannii |
33 |
12 |
| 3 |
Kocuria kristinae |
23 |
8.8 |
| 4 |
Candida tropicalis |
12 |
8.4 |
| 5 |
Klebziella pneumoniae |
22 |
4.5 |
| 6 |
Proteus mirabilis |
10 |
3.8 |
| 7 |
Methicillin- resistant Staphylococcus aureus (MRSA) |
9 |
3.4 |
| 8 |
Total infected Patients |
261 |
67.7 |
| |
Without any infection |
126 |
32.3 |
| |
Total Patients |
387 |
|
Table 1: Infection of microorganism at national burns centre (During study period).
| Concentration |
Volume of Shata Dhauta Ghruta/Jatyadi oil/ Oleuropein Oil/ Ozonated Oil (ml) |
Volume of Media (ml) |
Total Volume (ml) |
| 1:5 |
10 |
40 |
50 |
| 1:10 |
5 |
40 |
50 |
| Positive Control |
0 |
50 |
50 |
Table 2: Preparation of dilutions for agar plate with varing concentration used oil.
| Concentration |
Volume of Ozonated Oil (ml) |
Volume of Media (ml) |
Total Volume |
| 1:05 |
20 |
80 |
100 |
| 1:10 |
10 |
90 |
100 |
| 1:15 |
6.33 |
93.7 |
100 |
| 1:20 |
5 |
95 |
100 |
| 1:25 |
4 |
96 |
100 |
| 1:30 |
3.33 |
97.7 |
100 |
| 1:40 |
2.5 |
98.5 |
100 |
| 1:50 |
2 |
98 |
100 |
| 0.09375 |
1.33 |
98.7 |
100 |
| 0.111111111 |
1 |
99 |
100 |
| Positive Control |
0 |
100 |
100 |
Table 3: Preparation of dilutions for agar plate with varing concentration of oil.
| |
OD at 580nm |
| Time points in Hours |
MRSA 1 |
MRSA 2 |
MRSA 3 |
MRSA 4 |
MRSA 5 |
MRSA 6 |
| 0 |
0.14 |
0.08 |
0.13 |
0.08 |
0.12 |
0.12 |
| 2 |
0.2 |
0.27 |
0.38 |
0.19 |
0.21 |
0.2 |
| 4 |
2.18 |
1.37 |
1.62 |
1 |
0.7 |
1.34 |
| 6 |
3.2 |
3.09 |
2.7 |
1.76 |
1.5 |
2.89 |
| 8 |
4 |
3.14 |
2.7 |
2.66 |
2.12 |
2.89 |
| 10 |
4 |
3.17 |
2.81 |
3.29 |
2.67 |
3 |
Table 4: Optical density measured by spectrophotometer (for growth curve).
Results
Despite various therapeutic measures, treatments of MRSA remain to be a major challenge for Burns. To assess the significant effect of ozonated oil for MRSA infection, the in-vitro study carried out at National Burns Centre suggest that ozonated oil destroy the MRSA infection which were isolated from burn patients infection. At National Burns Centre, Airoli, Navi Mumbai, total number of 1679 burn patients were treated from December 2015 to September 2018 and we found 50 patients showing Methillicin Resistant S. aureus strain (MRSA) which account for 2.98 %. During the six month study period (September 2017-March 2018), a total of 387 burn wound samples were considered among which 261 samples were infected and those includes 152 Pseudomonas aeruginosa, 33 Acinetobacter baumannii, 23 Kocuria kristinae, 22 Klebziella pneumoniae, 12 Candida tropicalis, 9 Methicillin resistant Staphylococcus aureus (MRSA) and 126 samples were having no infections. The graph plotted in figure show the statistics of the microbiota of burn patients during 6 month period(Figure 2).
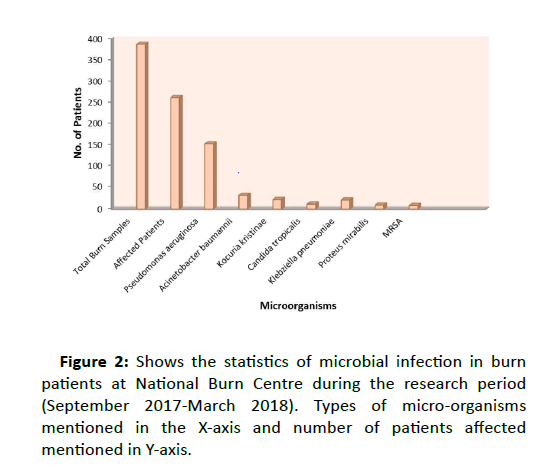
Figure 2:Shows the statistics of microbial infection in burn patients at National Burn Centre during the research period (September 2017-March 2018). Types of micro-organisms mentioned in the X-axis and number of patients affected mentioned in Y-axis.
Sub-culturing, Coagulase test and Growth Curve analysis
Six different MRSA randomly chosen and isolated colonies were sub-cultured in blood agar plate as shown in Figure 3. To characterize the Methicillin resistant strain, coagulase test was performed to check the agglutination property of MRSA. In the glass slide, a coarse clumping of cocci was visible to the naked eye within 10 seconds was read as positive. The absence of clumping or any reaction taking more than 10 seconds to develop was read as negative, but were re-examined for the tube coagulase test method. Clumping of MRSA bacteria were seen on glass slide and by test tube method, white jelly like mass is clearly visible in the test tube (Figure 4), that how the free coagulase enzymes of MRSAs are forming clumps. This result confirmed that in slide methods agglutination occurred by bound coagulase enzymes and in test tube method the free coagulase enzymes formed the clumps. For growth curve analysis, OD was taken in every 2 hours using Spectrophotometer at 580 nm setting the blank as zero. All the readings such as time as x value and absorbance as y value at for 10 hours. The graph was which takes the curve shape with three phases of bacterial growth – lag, log and stationary. Once we confirm about the strains of MRSA, we went ahead for using the natural compounds for their effective use against MRSA.

Figure 3:Sub-cultured MRSA colonies of six MRSA samples, showing isolated colonies of S.aureus (MRSA).

Figure 4:Coagulase Test (a) Slide method in which visible clumping of MRSA bacteria seen on glass slide indicates agglutination or clumping of bound coagulase enzyme. In figure (b) Test tube method where white jelly like mass is clearly visible in the test tube that how the free coagulase enzymes of MRSAs are forming clumps.
Novel Components used against MRSA growth
We have used the novel components such as (i) Shata Dhauta Ghruta; the ghee forms nano sized particles that enter the bacterial cell, which interfere with the various mechanisms in the bacterial cell and help in gradual elimination of the bacteria [11] (ii) Jatyadi Oil, which acts on the cell wall leading to formation of pores in the peptidoglycan layer and loss of essential components from within the cell. Thus it acts as a bactericidal agent [12, 13] (iii) Oleuropein; its antibacterial effect observed by damaging the bacterial membrane or disrupting cell peptidoglycans. Oleuropein are able to inhibit the development and production of enterotoxin B too [14] (iv) Ozonated Oil, which healing efficacy has been shown in combination with other drugs and the bactericidal effect has been observed. The other mechanism is based on oxidizing Poly Unsaturated Fatty Acids. Ozone acts as a general intracellular oxidant [15]. The effects of these compounds on the growth of MRSA were observed through Tissue culture plate method, when the growth-medium contain (A) Shat Dhyut Ghruth (B) Jatyadi Oil (C) Oleuropien oil (D) Ozonated oil. There was inhibition of growth of MRSA or no growth was seen in the plate where ozonated oil has been used in growth medium. However clear growth visible in rest all three components as shown in figure 5. For further investigation, to confirm the effect of ozonated oil, even much diluted form of ozonated oil was used (1:100) to make plate which can inhibit MRSA growth.
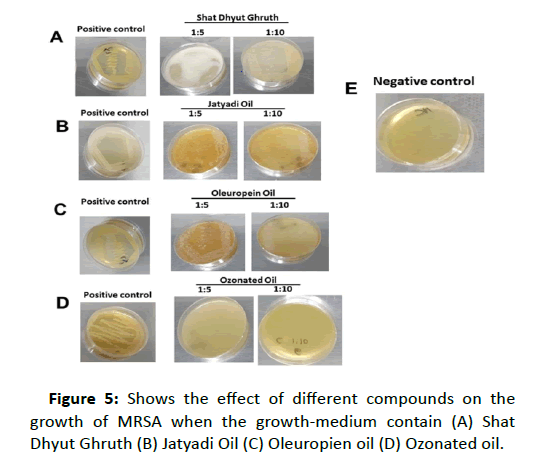
Figure 5:Shows the effect of different compounds on the growth of MRSA when the growth-medium contain (A) Shat Dhyut Ghruth (B) Jatyadi Oil (C) Oleuropien oil (D) Ozonated oil.
From the figure it can be clear that growth of MRSA with the dilution of 1:5 and 1:10 in growth medium were shown in the case of A, B and C. However, in figure D, where ozonated oil has been used in growth medium, there is no growth of MRSA in the plate. In comparison to the positive control (growth pattern) the compounds such as Shat Dhyut Ghruth, Jatyadi Oil and Oleuropien oil seems to have no effect on the growth of MRSA.
Cytocompatibility of Ozonated oil
The cytocompatibility of ozonized olive oil in human skin fibroblasts (HSFs) was evaluated intaking DMSO as positive control. The cells were incubated for 2 or 24 h with increasing dilution of ozonized oil and DMSO was treated as positive control. Alamar assay was done to check cell viability and proliferation. The percentage of viable cells was calculated relative to control cells and set to 100%. DMSO treated cells the percentage of non-viable cell indicated that ozonated oil is cytocompatible. The bright field image of skin fibroblasts before and after treatment is shown in figure 6.
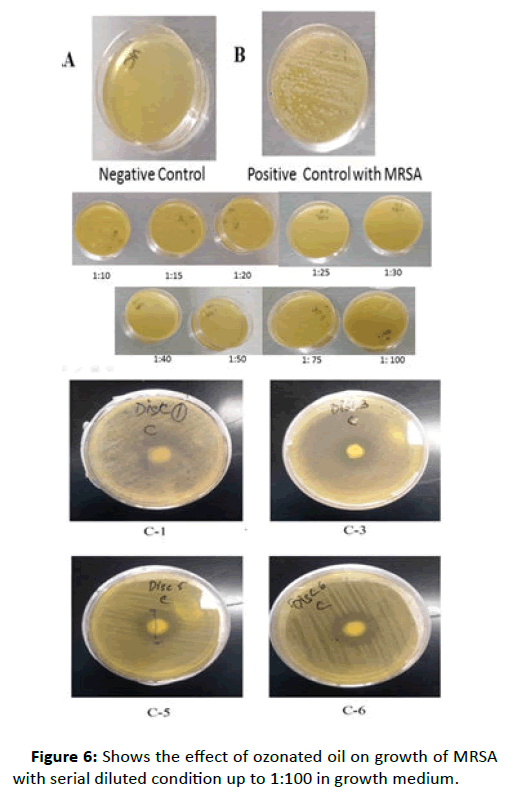
Figure 6:Shows the effect of ozonated oil on growth of MRSA with serial diluted condition up to 1:100 in growth medium.
This result clearly indicates that in lowest concentration of 1:100, ozonated oil inhibits the growth of MRSA. Figure 6A is the negative control which contain only medium, Figure 6B is positive control where MRSA colony looks very distinct and in figure C, all different dilutions of ozonated oil starting from higher (1:5) to lower 1:100 shows very clearly that there was not a single MRSA colony appeared even after 24-hour incubation.
Discussion and Conclusion
In burns a fast, effective and elimination of infection enhance wound healing process which substantially decrease medical costs and hospital stay. These factors significantly improve the burn patients' quality of life. However, overall morbidity and mortality of burn wound infections, tissue invasion, and secondary sepsis were extremely high during burn infections because the growth of bacteria on the burn wound surface are controlled but not eradicated. When antibiotics are administered orally or systemically (intravenously) either these drugs cannot reach the actual site of infection or relatively insufficient concentration [16]. For this reason various researchers and entrepreneurs are on the ongoing state of preparations and formulations of antibiotics for topical application and some of them are already been developed or occur naturally. There are wide varieties of natural compounds such as honey, Eucalyptus oil, Lavender oil, Tea tree oil, probiotics that have antimicrobial properties. However a few stands out from the rest in their ability to combat antibiotic resistant skin infections [17]. Methicillin-resistant Staphylococcus aureus (MRSA) is resistant to many different antibiotics and very contagious and can be spread through direct contact with an infected person [18]. Various antibacterial and antifungals have been used to eliminate the infection of MRSA. Consequently, the trial for killing of S. aureus is continuing and several nations are investing a lot of research for the same. These organisms in their stationary phase of growth were generally resistant to injury than those in the log phase. Since antimicrobial agents that affect cellulose synthetic processes often have little effect on organism in the stationary phase of growth. Ozonated oil prepared by ozonation of mixture of sesame, sunflower and other oils is being suggested to follow Criegee Mechanism which worked in the destruction of MRSA [19]. In Indian, the burn wound represented the susceptible site for opportunistic colonization by organisms of endogenous and exogenous origin. The observed cytocompatibility of ozonated oil in human fibroblast cell demonstrate that ozonized oil could be considered an alternative antibacterial agent. Ozonated oil, used in our study acts as antimicrobial weapons in the battle against S. aureus and other potential pathogens however, therapeutics derived from microorganisms themselves will offer promise as viable alternatives for treatment of MRSA too. To enhance our knowledge of the skin microbiota and use of Ozonated oil for MRSA treatment) will guide future research efforts directed towards elimination of MRSA and other microorganisms. The task of defeating the uncontrollable antibiotic resistance MRSA is challenging and it appears that to solve this problem will be impressive scientific creativity of researchers and entrepreneurs.
Acknowledgement
The approval of Medical Director for conducting this work at pathology department at National Burns Centre is highly acknowledged. Author thanks to management system of NBC for giving permission for spending expenditures from author’s salary.
Conflict of Interest
There is no conflict of interest.
37291
References
- Cook N (1998) Methicillin-resistant staphylococcus aureus versus the burn patient. Burns 24: 91-98.
- Glik J, Kawecki M, Gaździk T, Nowak M (2012) The impact of the types of microorganisms isolated from blood and wounds on the results of treatment in burn patients with sepsis. Pol Przegl Chir 84: 6-16.
- Pruitt BA, Wolf SE (2009) An historical perspective on advances in burn care over the past 100 years. Clin Plast Surg 36: 527-545.
- Edwards-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K (2004) The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 30: 772-777.
- Muthaiyan A, Biswas D, Crandall PG, Wilkinson BJ, Ricke SC (2012) Application of orange essential oil as an antistaphylococcal agent in a dressing model. BMC Complement Altern Med 12: 125.
- Azuma K, Mori T, Kawamoto K, Kuroda K, Tsuka T, et al. (2014) Anti-inflammatory effects of ozonated water in an experimental mouse model. Biomed Rep 2: 671-674.
- Pietrocola G, Ceci M, Preda F, Poggio C, Colombo M (2018) Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J Clin Exp Dent 10: e1103-e1108.
- Colombo M, Ceci M, Felisa E, Poggio C, Pietrocola G (2018) Cytotoxicity evaluation of a new ozonized olive oil. Eur J Dent 12: 585-589.
- Montevecchi M, Dorigo A, Cricca M, Checchi L (2013) Comparison of the antibacterial activity of an ozonated oil with chlorhexidine digluconate and povidone-iodine. A disk diffusion test. New Microbiol 36: 289-302.
- David H. Pinus, Microbial Identification using the Biomerieux VITEK2 Systems, Hazelwood, MO, USA
- Deshpande S, Deshpande A, Tupkari S, Agnihotri A (2009) Shata-dhauta-ghrita–A case study. Ind J Trad Knowl 8: 387-391.
- Dhande P, Simpy R, Kureshee N, Sanghavi DR, Pandit VA (2012) Burn wound healing potential of Jatyadi formulations in rats. Resr J Phar Biol Chem Sci 3: 747-753.
- Dev VS, Shailaja SV, Sharma V (2015) A Comparitive Clinical Study on the Effect of Jatyadi Taila in varicose ulcer and diabetic ulcer. Int Ayur Med J 3: 1950-1958.
- Omar HS (2010) Oleuropien in olive and it Pharmacological Effects. Sci Pharm 78: 133-154.
- Wysok B, Uradzinski J, Gomolka-Pawlicka M (2006) Ozone as an Alternative Disinfectant-A Review. Pol J food Nutri Sci. 15: 3-8.
- Sevgi M, Toklu A, Vecchio D, Hamblin MR (2013) Topical antimicrobials for burn infections-An update. Recent Pat Anti infect Drug Discov 8: 161-197.
- Honari S (2004) Topical therapies and antimicrobials in the management of burn wounds. Crit Care Nurs Clin North Am 16: 1-11.
- Glasser JS, Guymon CH, Mende K, Wolf SE, Hospenthal DR, et al. (2010) Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns 36: 1172–1184.
- Giorio C, Campbell SJ, Bruschi M, Archibald AT, Kalberer M (2017) Detection and identification of Criegee intermediates from the ozonolysis of biogenic and anthropogenic VOCs: comparison between experimental measurements and theoretical calculations. Faraday Discuss 200: 559-578.












