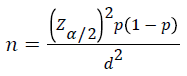Keywords
Cardiovascular disease; Metabolic syndrome; Diabetes
Introduction
Diabetes mellitus (DM) is a global problem, currently estimated more than 171 million individuals (>2.8% of the world’s population), and it is expected to rise 366 million (4.4% of the world’s population) and of this, >80% DM patients will be in developing countries by 2030 [1].
Metabolic syndrome (MetS) is a group of clinical and biological abnormalities that confers a greater risk of developing type II diabetes, cardiovascular diseases (CVD) and other related health problems [2]. Type II DM, is the one component of MetS which can leads the development of cardiovascular disease, and MetS is a cluster of metabolic disorders of numerous etiologies [3]. These include abdominal obesity, raised blood pressure, hyperglycemia and lipid profile derangement [3,4]. In addition various metabolic disorders were included with MetS later, such as micro-albuminuria, hyperuricemia (gout) and defects in fibrinolysis and blood coagulation [5]. Around 70-80% of the population with DM were diagnosed with the MetS [6,7]. Worldwide, 20-25% of the adults have been expected to have the MetS. Individuals with MetS are twofold as expected to die and three times as likely to have a heart attack or stroke when compared to people without MetS [8]. This indicates the co-existence effect of type II DM and MetS on cardiovascular risks [9]. Strictly diagnosing of MetS and its components in DM patients is a vital in order to promote patients health care and to minimize CVD related morbidity and mortality.
Data are available regarding the prevalence and rising trends of type II DM in Sub-Sahara. However, studies on the prevalence of the MetS among type-II DM patients are still inadequate in developing countries like Sub-Saharan Africa including Ethiopia. Hence, this study was aimed to assess the prevalence and risk factors of MetS among type-II DM patients in Hawassa University Comprehensive specialized Hospital, Southern Ethiopia.
Materials and Methods
Study setting and study population
A cross-sectional study was conducted among type II DM patients those who had regular follow-up at outpatient clinic of chronic diseases from March to November 2014, at Hawassa University comprehensive specialized Hospital, Southern Nations Nationalities and Peoples Region (SNNPR). Hawassa is the capital city of the region and located 275 km from Addis Ababa, which is the capital city of Ethiopia. The altitude of the town is 1697 meters above sea level with the mean annual temperature and rainfall of 20.9°C and 997.6 mm respectively. This hospital was established in November, 2005 and it serves for more than 10 million people and provides health care services for populations of SNNPR and neighboring regions. The study participants were previously diagnosed/ known type II DM patients; however patients who were on statin drugs, pregnant women, previously known heart failures and renal failures were excluded from the study.
Sample size and sampling technique
Sample size calculation was based on the prevalence of MetS in Ghanaian Type II DM patients, which was 24% [10]. The required sample size was computed using single population proportion formula,
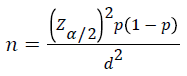
Where, P= proportion of MetS, Zα/2= Critical value at 95% level of confidence (Z =1.96) d=Margin of error (5%). Based on the above formula the final sample size was 281. Then, systematic random sampling technique was applied to select the study participants from the study populations.
Data collection
Socio-demographic, clinical and anthropometric data collection: The socio-demographic, clinical and anthropometric related data of the study participants were collected using structured questionnaire including with review of patients clinical records.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) was measured from each subject with a standard adult arm cuff (sphygmomanometer). Trained nurses who were working in the outpatient chronic diseases follow-up clinic were done the measurement from right hand after patients rested for at least 5 minutes. The precision of the measurement was checked by using two readings within two minutes difference and finally the average of the two readings was taken as blood pressure. Data regarding weight (in Kilogram (kg) and to the nearest 0.1 kg), height (in meters (m) and to the nearest 0.1 m) and waist circumference (in centimeter (cm) and to the nearest 0.1 cm) were collected according to WHO STEPS manual [11] using appropriate equipments as well as measuring scales. Height of the participants was measured without wearing shoes and weight was taken with wearing light garments. In addition waist circumference was measured with measuring tape at the midpoint between the inferior angle of rib and the iliac crest at the end of a relaxed expiration so far patients were upright properly. Furthermore body mass index (BMI: kg/m2) was calculated by dividing individual’s weight (kg) by height squared (m2).
Blood collection and serum biochemical determination
About 4-5 milliliters (ml) of venous blood sample was drawn from each study participant after overnight fasting and centrifuged at 3000 cycles/min, and then serum was obtained. Also serum samples were analyzed for FBS and lipid profile (total cholesterol (TC), high density lipoprotein cholesterol (HDL-c) and TGs) using A25TM BioSystem Random Access chemistry analyzer. Serum TC, TGs and FBS were analyzed using enzymatic colorimetric assay method (Linear chemicals, Montgat, Spain). Serum HDL-c determined by enzymatic colorimetric assay method after a selective precipitation of apolipoprotein containing lipoproteins (very low density lipoprotein cholesterol (VLDL-c) low density lipoprotein cholesterol (LDL-c), and apolipoprotein a (Lpa)) by phosphotungstic acid/MgCl2 (magnesium chloride) (Linear chemicals, Montgat, Spain).
Definition of metabolic syndrome
Finally MetS was defined according to modified United States National Cholesterol Education Program, Adult Treatment Panel (NCEP-ATP) III guideline. Patients had at least three of the following risk features to be categorized under the group of MetS: abdominal obesity (defined as waist circumference >102 cm in males and >88 cm in females); elevated TGs (≥ 150 mg/dl); low HDL-c (<40 mg/dl in males and <50 mg/dl in females); elevated blood pressure (SBP ≥ 130 or DBP ≥ 85 mmHg) and FBS >110 mg/dl [12].
Data analysis
All questionnaires were checked visually, coded and entered into Statistical Package for Social Sciences (SPSS) Version 20 for analysis. To explain study population in relation to appropriate variables descriptive statistics were used. Chi-square was used for categorical variables. In addition, evaluation of differences in means of study groups was evaluated using student’s t-test. In addition, bivariate and multivariate binary logistic regression models were used to assess the differences in the distribution of categorical variables in the study groups. Furthermore, P-value <0.2 was used as a cut-off value to include variables for multivariate binary logistic regression and at 95% confidence interval (CI), a P value <0.05 was accepted as statistically significant.
Data quality control
Nurses working in the outpatient chronic diseases follow-up clinic were trained concerning socio-demographic, clinical and physical/anthropometric data collection from the study participants. All laboratory investigations were performed by laboratory technologists using standard operating procedures (SOPs). A All analytical phase of lab performances as well as reagents condition were checked daily by performing the internal quality control samples. If results fall outside established value, the run was repeated and also recalibration of instrument was done when needed.
Ethical concern
The ethical clearance of study was obtained from Institutional Review Board of the College of Medicine and Health Sciences, Hawassa University. Then official letter was submitted to hospital medical director office and data collection was performed after obtaining permission. Participation was completely voluntary, and written informed consent was obtained from all the study subjects after indepth clarification about the objective of the study. Every information obtained throughout the study was kept with confidentiality and all laboratory analysis was performed without any charge.
Results
Background characteristics of the study subjects
Totally 281 type II DM participants were approached; of these 270 patients [166 (61.5%) males and 104(38.5%) females] were recruited in the study, with a response rate of 96.1%. The mean and standard deviation (±SD) age of the participants was 48.8 (±11.9) years and males were significantly older than females (p=0.04). About, 55.2% were urban dwellers with a high proportion of females (p=0.008). About 94.1%, 35%, and 76.7% of the study participants were married, employed, and majority time uses foot for transportation, respectively. Also 54.8% of the study participants have no income (like a housewife and older patients) and they depend on their husbands and their family (Table 1).
Table 1 Socio-demographic and other characteristics of type II diabetes patients at Hawassa University comprehensive specialized hospital.
| Variables |
|
Total 270(%) |
Men 166(%) |
Women
104(%) |
p-value |
| Mean Age, years (SD) |
|
48.8(11.9) |
50(12.4) |
46.9(10.9) |
0.04 |
| <45 |
96(35.6) |
56(33.7) |
40 (35.6) |
|
| 45-54 |
85(31.5) |
52(31.3) |
33(31.7) |
|
| 55-64 |
59(21.9) |
37(22.3) |
22(21.2) |
|
| ≥65 |
30(11.1) |
21(12.7) |
9(8.7) |
0.71 |
| Residence: |
Rural |
121(44.8) |
85(51.2) |
36(34.6) |
|
| Urban |
149(55.2) |
81(48.8) |
68(65.4) |
0.008 |
| Marital status: |
Single |
12(4.4) |
10(6.0) |
2(1.9) |
|
| Married |
254(94.1) |
154(92.8) |
100(96.2) |
|
| Divorced |
2(0.7) |
1(0.6) |
1(0.96) |
|
| Widow/widower |
2(0.7) |
1(0.6) |
1(0.96) |
0.44 |
| Educational status |
Unable to read and write |
69(25.6) |
27(16.3) |
42(40.4) |
|
| Primary |
67(24.8) |
49(29.5) |
18(17.3) |
|
| Secondary |
67(24.8) |
40(24.1) |
27(26.0) |
|
| Tertiary |
67(24.8) |
50(30.1) |
17(16.3) |
<0.0001 |
| Occupation |
Farmer |
68(25.2) |
64(38.6) |
4(3.8) |
|
| Employed |
96(35.6) |
70(42.2) |
26(25.0) |
|
| Merchant |
27(10.0) |
25(15.1) |
2(1.9) |
|
| Housewife |
68(25.2) |
0(0.0) |
68(25.2) |
|
| Daily laborers &Others |
11(4.1) |
7(4.2) |
4(3.8) |
<0.0001 |
| Mode of transport |
foot |
207(76.7) |
126(75.9) |
81(77.9) |
|
| Bicycle/cart |
19(7.0) |
17(10.2) |
2(1.9) |
|
| Motor vehicle |
44(16.3) |
23(13.9) |
21(20.2) |
0.02 |
| *Monthly income |
No income (dependent) |
148(54.8) |
82(49.4) |
66(63.5) |
|
| ≤ 1000 |
45(16.7) |
29(17.5) |
16(15.4) |
|
| 1001-2000 |
55(20.4) |
39(23.5) |
16(15.4) |
|
| 2001-3000 |
15(5.6) |
11(6.6) |
4(3.8) |
|
| ≥3001 |
7(2.6) |
5(3.0) |
2(1.9) |
0.22 |
*Income, in Ethiopian birr
Clinical and other characteristics of the study subjects
About 92.6% of patients were ≥1year since the diagnosis of diabetes. Mean BMI and HDL-c were significantly higher in females when compared to males. Both an overweight and obese participants accounted 45.2%, and females were more obese when compared to males (22.1% vs. 4.8%; P <0.0001), respectively. In addition, 2.6% and 6.7% were ever smoked cigarette and ever drunk alcohol respectively. Oral blood glucose lowering agents have been taken by 51.9% participants; whereas 45.9% were on insulin injection and 2.2% patients were on combined treatment (oral agents and insulin injection) (Table 2).
Table 2 Clinical and other characteristics of type II diabetes patients at Hawassa University comprehensive specialized hospital.
| Variables |
|
Total 270 |
Men N=166 |
Women
N=104 |
p-value |
| Duration since the diagnosis of DM |
<1 years |
20 (7.4) |
15(9.0) |
5(4.8) |
0.16 |
| 1-5 years |
169(62.6) |
108(65.1) |
61(58.7) |
| 6-10 years |
58(21.5) |
29(17.5) |
29(27.9) |
| ≥ 11 years |
23(8.5) |
14(8.4) |
9(8.7) |
| Mean BMI, Kg/m2(SD) |
|
24.5(4.3) |
23.8(3.7) |
25.7(5.0) |
<0.0001 |
| |
<18.5 kg/m2 |
19(7.0) |
12(7.2) |
7(6.7) |
<0.0001 |
| 18.5-24.9 kg/m2 |
128(47.4) |
88(53.0) |
40(38.5) |
| 25-29.9 kg/m2 |
92(34.1) |
58(34.9) |
34(32.7) |
| ≥30 kg/m2 |
31(11.1) |
8(4.8) |
23(22.1) |
| Mean WC, cm(SD) |
|
95.5(11.1) |
94.9(10.0) |
96.3(12.3) |
0.33 |
| Mean Hip cm(SD) |
|
99(9.7) |
97(8.2) |
102(11.0) |
<0.0001 |
| Mean WC to Hip ratio(SD) |
|
0.96(0.06) |
0.98(0.06) |
0.94(0.06) |
<0.0001 |
| Mean SBP, mmHg(SD) |
|
125(17.2) |
125.9(16.2) |
123.9(18.8) |
0.34 |
| Mean DBP, mmHg(SD) |
|
79.6(9.7) |
80(9.7) |
78(9.6) |
0.4 |
| Median FBS, mg/dl(SD) |
|
80(70-203) |
150(110-212) |
156(117-215) |
0.86 |
| Mean HDL, mg/dl(SD) |
|
45.1(13.6) |
43.2(14.5) |
48.1(11.4) |
0.005 |
| Median TGs, mg/dl (IQR) |
|
176(134-234) |
179(136-244) |
172(132-223) |
0.24 |
| Ever smocked cigarette |
|
7(2.6) |
6(3.6) |
1(0.96) |
0.18* |
| Ever drank alcohol |
|
18(6.7) |
15(9.0) |
3(2.9) |
0.05* |
| Treatmentsfor DM: |
Oral agents |
140(51.9) |
86(51.8) |
54(51.9) |
|
| Insulin |
124(45.9) |
75(45.2) |
49(47.1) |
|
| Oral agents + insulin |
6(2.2) |
5(3.0) |
1(0.96) |
0.53* |
| DM treatment combination |
single |
202(74.8) |
120(72.3) |
82(78.8) |
|
| Combined |
68(25.2) |
46(27.7) |
22(21.2) |
0.23 |
BMI:Body mass index; DBP: Diastolic blood pressure; DM:Diabetes mellitus FBS:Fasting blood sugar;IQR: Interquartile range; HDL-c:High density lipoprotein cholesterol; SD: Standard deviation; WC:Waist circumference; SBP: Systolic blood pressure; TGs: Triglycerides; *: significance by fishery exact test
Characteristics of metabolic syndrome and its components
According to the modified NCEP-ATP III criteria, the overall 45.9% of the study participants had MetS, and the proportion was significantly higher among females when compared to males (60.6% vs. 36.7%; p<0.0001), respectively. The frequency of MetS components among the participants were: abdominal obesity, raised blood pressure, raised TGs and low HDL-c were 40.7%, 28.1%, 68.1% and 47%, respectively. In addition, the prevalence of abdominal obesity and low HDL-c were significantly higher in females when compared to males (P ≤ 0.0001) for both MetS components (Table 3). Moreover, the proportion of three, four and five MetS components abnormal within a single individual were 26.3%, 16.3% and 3.7%, respectively (Figure 1).
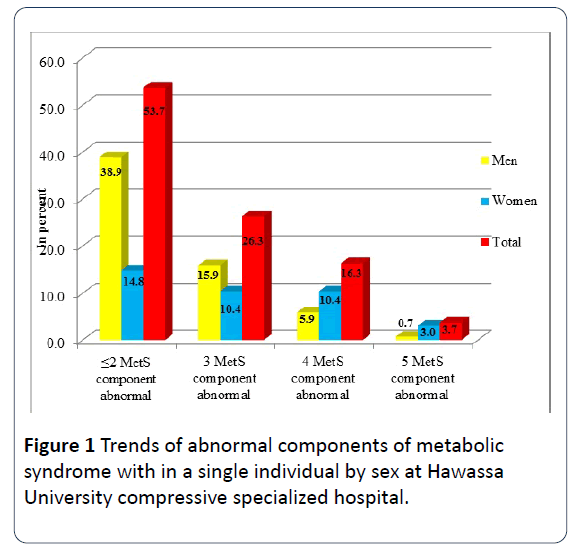
Figure 1: Trends of abnormal components of metabolic syndrome with in a single individual by sex at Hawassa University compressive specialized hospital.
Table 3 Prevalence of metabolic syndrome and its components other than hyperglycemia among type II diabetes patients at Hawassa University compressive specialized hospital.
| Variable |
Total 270 (%) |
Men1 66(%) |
Women 104(%) |
p-value |
| Metabolic syndrome |
124(45.9) |
61(36.7) |
63(60.6) |
<0.0001 |
| Abdominal obesity |
110(40.7) |
35(21.1) |
75(72.1) |
<0.0001 |
| Raised triglycerides |
184(68.1) |
113(68.1) |
71(68.3) |
0.97 |
| Reduced HDL-c |
127(47.0) |
63(38.0) |
64(61.5) |
<0.0001 |
| Raised blood pressure |
76(28.1) |
48(28.9) |
28(26.9) |
0.78 |
Factors associated with the prevalence of metabolic syndrome
Bivariate analysis was applied to assess the independent risk factors for MetS. Therefore being a female the crude odds ratio and 95% confidence interval [COR (95% CI): 2.6(1.6-4.4)], duration since the diagnosis of diabetes from 1-5years [COR (95% CI): 4.6(1.3-16.4)], and duration of diabetes ≥ 6 years [COR (95% CI): 7.1(1.9-26.1)] were significantly associated with MetS. In addition, being urban dweller [COR (95% CI): 1.8(1.1-2.9)], overweight [COR (95% CI): 12.1(2.6-55.4) and obesity [COR (95% CI): 29.1(5.4-157.9)] were also significantly and positively associated with MetS.
However, multivariate analysis was adjusted for potential confounding factors, being female [AOR (95% CI): 4.1 (1.5-10.9)], duration of DM since its diagnosis from 1-5 years [AOR (95% CI): 5.5 (1.3-24.0)] and duration of DM ≥ 6 years [AOR (95% CI): 7.3 (1.6-32.7)] were significantly associated with MetS. Further, overweight and obesity were also significantly and positively associated with MetS (Table 4).
Table 4 Factors associated with metabolic syndrome among type II diabetes patients at Hawassa University comprehensive specialized hospital.
| Variables |
MetSn(%) |
|
|
|
|
| Yes |
No |
COR (95% CI) |
p-value |
AOR (95% CI) |
p-value |
| Gender |
Men |
61(22.6) |
105(38.9) |
1 |
|
1 |
|
| women |
63(23.3) |
41(15.2) |
2.6(1.6-4.4) |
<0.0001 |
4.1(1.5-10.9) |
0.005 |
| Age, years |
<45 |
38(14.1) |
58(21.5) |
1 |
|
1 |
|
| 45-54 |
44(16.3) |
41(15.2) |
1.64(0.91-2.9) |
0.1 |
1.9(0.93-3.89) |
0.08 |
| 55-64 |
29(10.7) |
30(11.1) |
1.5(0.76-2.84) |
0.24 |
1.9(0.85-4.3) |
0.11 |
| ≥65 |
13(4.8) |
17(6.3) |
1.2(0.51-2.68) |
0.72 |
1.58(0.58-4.3) |
0.37 |
| Residence |
Rural |
46(17.0) |
75(27.8) |
1 |
|
1 |
|
| Urban |
78(28.9) |
71(26.3) |
1.8(1.1-2.9) |
0.02 |
1.2(0.64-2.38) |
0.53 |
| Marital status |
Single |
3(1.1) |
9(3.3) |
1 |
|
1 |
|
| Married |
119(44.1) |
135(50.0) |
2.6(0.7-9.9) |
0.15 |
0.9(0.19-4.27) |
0.89 |
| Divorced/Widow |
2(0.7) |
2(0.7) |
3.0(0.28-31.6) |
0.36 |
0.29(0.01-5.9) |
0.42 |
| Occupation |
Farmer |
26(9.6) |
42(15.6) |
1 |
|
1 |
|
| Employed |
48(17.8) |
48(17.8) |
1.6(0.86-3.0) |
0.14 |
0.75(0.31-1.8) |
0.52 |
| Merchant |
10(3.7) |
17(6.3) |
0.95(0.38-2.4) |
0.91 |
0.54(0.17-1.77) |
0.31 |
| Housewife |
37(13.7) |
31(11.5) |
1.9 (0.97-3.8) |
0.06 |
0.31(0.09-1.1) |
0.07 |
| Others |
3(1.1) |
8(3.0) |
0.61(0.15-2.5) |
0.49 |
0.27(0.04-1.6) |
0.15 |
| Duration of DM since its diagnosis, years |
<1 |
3(1.1) |
17(6.3) |
1 |
|
1 |
|
| 01-May |
76(28.1) |
93(34.4) |
4.6(1.3-16.4) |
0.02 |
5.5(1.3-24.0) |
0.02 |
| ≥6 |
45(16.7) |
36(13.3) |
7.1(1.9-26.1) |
0.003 |
7.3(1.6-32.7) |
0.009 |
| Mode of transportation |
Foot |
88(32.6) |
119(44.1) |
1 |
|
1 |
|
| Cart/Bicycle |
8(3.0) |
11(4.1) |
0.98(0.38-2.5) |
0.97 |
1.2(0.36-3.74) |
0.79 |
| Motor vehicle |
28(10.4) |
16(5.9) |
2.4(1.2-4.6) |
0.01 |
1.8(0.77-4.3) |
0.17 |
| *Monthly income |
No income |
68(25.2) |
80(29.6) |
1 |
|
1 |
|
| ≤ 1000 |
19(7.0) |
26(9.6) |
0.9(0.44-1.7) |
0.66 |
1.0(0.44-2.3) |
0.98 |
| 1001-2000 |
24(8.9) |
31(11.5) |
0.9(0.45-1.69) |
0.77 |
0.54(0.22-1.33) |
0.18 |
| 2001-3000 |
10(3.7) |
5(1.9) |
2.4(0.77-7.2) |
0.13 |
3.1(0.77-13.0) |
0.11 |
| ≥3001 |
3(1.1) |
4(1.5) |
0.91(0.19-4.1) |
0.87 |
0.77(0.12-4.97) |
0.78 |
| History ofalcoholism, n (%) |
No |
199(44.1) |
133(49.3) |
1 |
|
1 |
|
| Yes |
5(1.9) |
13(4.8) |
0.43(0.15-1.24) |
0.12 |
0.48(0.13-1.77) |
0.27 |
| BMI, Kg/m2 |
<18.5 |
2(0.7) |
17(6.3) |
1 |
|
1 |
|
| 18.5-24.9 |
44(16.3) |
84(31.1) |
4.4(0.98-20.1) |
0.05 |
4.1(0.84-20.5) |
0.08 |
| 25-29.9 |
54(20.0) |
38(14.1) |
12.1(2.6-55.4) |
0.001 |
12.0(2.3-61.4) |
0.003 |
| ≥30 |
24(8.9) |
7(2.6) |
29.1(5.4-157.9) |
<0.0001 |
19.8(3.1-124.7) |
0.001 |
DM:Diabetes; BMI:Body mass index; COR: Crude odds ratio; AOR: Adjusted odds ratio; CI: Confidence Interval; *: Income in Ethiopian birr; MetS: Metabolic syndrome
Discussion
Different studies in Africa settings reported that varies prevalence’s of MetS, which ranges from 51% - 60.4% [9,13-16]. However we found that the total prevalence of MetS among type II DM patients was 45.9% according to the modified NCEP-ATP III criteria. The percentage in MetS variation may be attributed to socio-demographic, individuals’ lifestyle and genetic variances among our population and the above mentioned studies. In addition, one study from another Africa setting reported that 43% of MetS among DM patients and which is comparable with our finding. Furthermore, the lower prevalence rate of MetS reported from Ghana diabetic patients (24.0%) [10] and Nigeria patients (25.2%) [17], when compared to the finding of present study. The variation could be due to applying different guideline to define MetS: accordingly the above mentioned two studies were used IDF and WHO criteria; whereas we used NCEP-ATP III guideline.
Our study indicated that females have significantly high proportion of MetS when compared to males (60.6% vs. 36.7%; p ≤ 0.0001), respectively. The finding is in line with different studies report including with Sub-Saharan countries [9,10,14,18]. In addition one study revealed that the proportion of MetS was 66.8% in males and 87.1% in females [19] and the rate is higher than the present study.
This study showed that high prevalence of raised TGs level (68.1%) and studies suggest that the co-existence of elevated TGs and low HDL-c are the risk factors for developing coronary heart diseases (CHD) [20,21]. In addition studies reported that low HDL-c level and abdominal obesity were significantly higher components of MetS in females when compared to males and the finding is similar with the report of our study [16,22,23]. The low concentration of HDL-c favors the accumulation of LDL-c in the blood vessels greatly due to its poor scavenging capacity of LDL-c from the body and this initiates the risks of CVD. Furthermore it is well-known that low concentration of HDL-c level is a strong independent risk factor for CHD [24,25].
This study showed that overweight and obesity were significantly associated with the prevalence of MetS in both logistic regression models. The finding is in line with the report of different studies [10,16,18]. In addition the present study showed that being a female was risk factor for developing MetS. Similarly several studies reported that females were at a high risk of increasing MetS and it may consequences CVD [16,26,27]. This could be attributed to genetic variation, due to their lifestyle and may be women doing light jobs. Furthermore we found that the increased duration since the diagnosis of diabetes was significantly associated with MetS. In contrary to our finding, one study revealed that no association of MetS with the duration of diabetes [28]. The discrepancy may be due to lack of knowledge and awareness regarding MetS in the current study and the educational status might have a great influence (because more than half of our study participants were ≤ primary level).
Limitation of the Study
We didn’t perform the nutritional assessment due to its difficulties; but the increased risk of cardiovascular diseases associated with defined MetS and its components is well known. The other limitations were the cross-sectional nature of the study and only one definition was used to assess MetS; a different prevalence rate could have been seen if other definition likes, IDF and WHO were used. Irrespective of these restrictions, the study ultimately adds evidence to the limited data.
Conclusion
Type II DM patients had high prevalence of MetS and elevated TGs was the most commonly encountered component of MetS. Abdominal obesity and low HDL-c were significantly higher in females compared to males. Being an overweight, being an obese, being a women and duration since the diagnosis of diabetes were significantly associated with occurrence of MetS. Therefore all patients should get awareness concerning MetS and screened for the presence of cardiovascular risk factors that contribute to the occurrences of MetS. Similarly applying comprehensive approach in each components of MetS management (like lifestyle and diet intake modification, doing regular moderate to vigorous intensity of physical exercises and using pharmacotherapies) is also critical to minimize risks of CVD related morbidity and mortality. Furthermore cohort studies should be done to address other predictors of MetS.
Acknowledgement
We strongly acknowledge the nurses who were working in the outpatient chronic diseases follow-up clinic and medical laboratory technicians/technologists of Hawassa University comprehensive specialized hospital for their endless support throughout data collection. Our appreciation is also protracted to the type II diabetic patients for their willingly participation in the study.
Funding
This study was financially supported by Hawassa University.
Authors’ Contributions
AT and HA conceived and designed the study, performed data collection, data entry and analysis including with interpretation and manuscript preparation, DA assisted in analysis and manuscript appraisal. All authors read and accepted the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
19580
References
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047-1053.
- Zimmet P, McCarty DJ, de Courten MP (1997) The global epidemiology of noninsulin-dependent diabetes mellitus and the metabolic syndrome. JDiabetes Complications 11:60-68.
- World Health Organization (1999) Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation, part I: Diagnosis and classification of diabetes mellitus. Geneva, Switzerland.
- International Diabetes Federation (2015) The IDF consensus worldwide definition of the metabolic syndrome.
- Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24: 683-689.
- Marchesini G, Forlani G, Cerrelli F, Manini R, Natale S, et al. (2004) WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with type 2 diabetes. Diabet Med 21:383-387.
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, et al. (1998) Prevalence of insulin resistance in metabolic disorders: The Bruneck study. Diabetes 47:1643-1649.
- Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM (2004) Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 27:2676-2681.
- Kengne AP, Limen SN, Sobngwi E, Djouogo CF, Nouedoui C (2012) Metabolic syndrome in type 2 diabetes: comparative prevalence according to two sets of diagnostic criteria in sub-Saharan Africans. DiabetolMetabSyndr 4:22-24.
- Salifu ZSMV, Abedandi R (2014) Prevalence, components and associated demographic and lifestyle factors of the metabolic syndrome in type 2 diabetes mellitus. J Diabetes MetabDisord13:80.
- World Health Organization (2017) Chronic diseases and health promotion: Stepwise approach to surveillance (STEPS).
- National Cholesterol Education Program (NCEP)(2002)The third report of the National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106:3143-3421.
- Adediran O, Edo A, Jimoh A, Ohwovoriole A (2007) Prevalence of the metabolic syndrome among Nigerians with type 2 diabetes. Diabetes Int 15:13-15.
- Isezuo S, Ezunu E (2005) Demographic and clinical correlates of metabolic syndrome in Native African type-2 diabetic patients. J Natl Med Assoc 97:557.
- Isezuo S (2005) Is high density lipoprotein cholesterol useful in diagnosis of metabolic syndrome in native Africans with type 2 diabetes? Ethn Dis 15:6.
- Nsiah K, Shang VO, Boateng KA, Mensah FO (2015) Prevalence of metabolic syndrome in type 2 diabetes mellitus patients. Int J Appl Basic Med Res 5:133-138.
- Alebiosu CO, Odusan BO (2004) Metabolic syndrome in subjects with type-2 diabetes mellitus. J Natl Med Assoc 96:817.
- Ogbera AO (2010) Prevalence and gender distribution of the metabolic syndrome. DiabetolMetabSyndr2:4.
- Kelliny C, William J, Riesen W, Paccaud F, Bovet P(2008) Metabolic syndrome according to different definitions in a rapidly developing country of the African region. CardiovascDiabetol 7:27.
- Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR (2001) Influence of low high–density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation 104:3046-3051.
- Jeppesen J, Hein HO, Suadicani P, Gyntelberg F (1997) Relation of high TG-Iow HOL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. ArteriosclerThrombVascBiol 17:1114-1120.
- Lee YJ, Tsai JC (2002) ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in a Chinese type 2 diabetic patients. Diabetes Care 25: 1002-1008.
- Ahmed A, Ahmad T, Hussain SJ, Javed M(2010) Frequency of metabolic syndrome in patients with Type-2 diabetes. J Ayub Med Coll Abbottabad 22: 139-142.
- Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM (2000) Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 342:1392-1398.
- Chapman MJ (206) Therapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart disease. PharmacolTher 3:893-908.
- FelixVal K, Titty WK, Owiredu WK, AgyeiFrimpong MT (2008) Prevalence of metabolic syndrome and its components among diabetes patients in Ghana. J BiolSci 8:1057-1061.
- Ford ES, Giles WH, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 287:356-359.
- Shimajiri Y, Tsunoda K, Furuta M, Kadoya Y, Yamada S, et al.(2008) Prevalence of metabolic syndrome in Japanese type 2 diabetic patients and its significance for chronic vascular complications. Diabetes Res ClinPract 79:310–317.