Case Report - (2022) Volume 13, Issue 5
Pediatric neuromyelitis optica and it management in rural practice in north Togo: About a 13-year-old young girl and review of literature
Léhleng Agba1*,
Nyinèvi Anayo2,
Lihanimpo Djalogue3,
Massaga Dagbe4,
Kokou Mensah Guinhouya2,
Vinyo Kumako1,
Damelan Kombate5,
Kossivi Apetse2,
Abide Talabewi2,
Komi Assogba5,
Mofou Belo2 and
Ayelola Balogou6
1Department of Neurology, University Hospital Center of Kara, Kara, Togo
2Department of Neurology, University Hospital Center of Lomé, Lomé, Togo
3Department of Internal Medicine, University Hospital Center of Kara, Kara, Togo
4Department of Radiology, University Hospital Center of Kara, Kara, Togo
5Department of Neurology, Regional Hospital Center of Kara, Kara, Togo
6Department of Neurology, University Hospital Center of Campus, Lomé, Togo
*Correspondence:
Léhleng Agba, Department of Neurology, University Hospital Center of Kara,
PoBox 618 Kara,
Togo,
Email:
Received: 07-May-2022, Manuscript No. ipjnn-22-12777;
Editor assigned: 09-May-2022, Pre QC No. P-12777;
Reviewed: 19-May-2022, QC No. Q-12777;
Revised: 25-May-2022, Manuscript No. R-12777;
Published:
31-May-2022
Abstract
Considered for a long time as a particular form of multiple sclerosis
(MS), Devic's disease generally occurs in young women between
20 and 40 years old. This neuro-immunological disease can affect
children and adolescents. It was the case of a 13-year-old student
who came to the clinic with a walking disorder of insidious onset
and ascending progression. Guillain-Barré syndrome was the first
diagnosis retained but when visual troubles occurred, the medullary
MRI and the search for anti Aquaporine-4 (AQP-4) antibodies where
done. The medullary MRI showed a hypersignal in T2 weighted
extended on 6 vertebrae at the cervical level. The anti-AQP-4
antibody was positive. Devic's disease was then recognized in this
young girl who was given intravenous (IV) corticosteroid. The lack
of the therapeutic arsenal in our region did not allow the patient to
receive plasma exchange and IV immunoglobulin as recommended
by the guidelines. However, with corticosteroid therapy at 30
mg/kg/day for 5 days, the neurological disorders stabilized. With
Azathioprine as preventive treatment and physiotherapy, the
patient improved after 3 months, without complete recovery.
Keywords
Devic's neuromyelitis optica; Childhood; Togo; Sub-
Saharan Africa
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an
uncommon inflammatory disease of the central nervous
system, manifesting clinically as optic neuritis (ON),
myelitis, and certain brain and brainstem syndromes [1].
For a longtime, Devic's disease was considered a special
form of multiple sclerosis (MS) and treated as such [2].
Neuromyelitis optica (NMO) usually occurs in young
women between the ages of 20 and 40 [3]. However, it can
occur in children and teenagers. The recent discovery of
anti-aquaporin-4 (AQP-4) antibodies has led to advances in
the early diagnosis of this disease regardless of the patient's
age. We report the case of a 13-year-old female student who
was seen in a neurology department. Through this case we
will review the literature on the diagnostic and therapeutic
particularities of pediatric forms of NMO.
Case Description
A 13-years old student girl presented to neurology
department of CHU-Kara for a walking disorder started
insidiously and worsened over about 3 weeks. Symptoms
started with bilateral and symmetrical sensory and motor
disorders of the lower limbs followed by an upward
progression from toes to roots of lower limbs within a week.
This presentation suggested an acute polyradiculoneuritis
and a lumbar puncture was performed. The latter revealed
an albuminocytological dissociation on examination of
cerebrospinal fluid (CSF). She was treated in internal
medicine with intravenous (IV) corticosteroids for 3 days
and was then referred for physiotherapy. The symptoms
worsened within 2 weeks, marked by an increase in sensory
disorders of the trunk where they were belt-like. These new
symptoms were associated with a decrease in visual acuity,
which led to a referral to neurology. Her personal and
family medical history is unremarkable. On examination,
she was in good general condition and weighed 28 kg for a
height of 1.35 m. She was in a good state of consciousness.
She had no other upper functional disorders. There was a
symmetrical paraparesis that predominated in the distal
parts of the limbs. The muscle strength score was 0/5 for
ankle flexion-extension, 3/5 for knee flexion-extension
and 4/5 for hip flexion. There was no amyotrophy or fasciculation. Deep tendon reflexes of the lower limbs were
sharp with ankle tremor more marked on the right than
on the left. The plantar reflexes test showed Babinski sign
on both sides. There was no motor trouble in upper limbs.
Sensory examination showed superficial and deep sensory
involvement in the lower limbs extending up to the trunk
with a clear sensory level at D6 and a band of hypoesthesia
from D6 to D4. On examination of the cranial nerves,
there was a decrease in visual acuity; the other cranial
nerves were unaffected. She had urinary incontinence.
There was no saddle hypesthesia or anesthesia on perineal
touch. Cardiopulmonary examination showed regular
heart sounds and a eupneic breathing. Spine examination
was normal. The association of the spinal cord syndrome
and the decrease in visual acuity led to the suggestion of
neuromyelitis optica, from which an ophthalmological
consultation was requested. The latter confirmed the
decrease in visual acuity without any pathological sign
on ophthalmoscopy and on a slit lamp examination. The
diagnosis of retrobulbar neuritis was made following this
ophthalmological consultation. The blood cell count
revealed a biological inflammatory syndrome with a
sedimentation rate of 50 mm and a C-Reactive Protein
(CRP) of 36 mg/l. Renal and hepatic functions were
normal. Human immunodeficiency (HIV) serology was
negative. CSF examination showed hyperproteinorrachia
at 1.7g/l with normal glucorarrachia and chlorurrachia.
Magnetic resonance imaging (MRI) of the spinal cord
showed extensive hypersignal over 6 vertebral bodies in the cervical region on the T2 sequence (Fig. 1). Brain
MRI was normal. AQP-4 was found to be positive and
anti-myelin olygodendrocyte glycoprotein (anti MOG)
negative. The diagnosis of Devic's neuromyelitis optica was
made, and the patient was given IV corticosteroid at 840
mg of methylprednisolone in the day for 5 days associated.
This corticotherapy was combined with adjuvant treatment
with potassium, calcium, omeprazole and antibiotic
therapy to prevent a urinary tract infection. Physiotherapy
to support the drug treatment was instituted. The patient
has well improved clinically with a recovery of muscle
strength in the lower limbs allowing walking with a walker.
Azathioprine 25 mg orally every 12 hours was given in
addition to the corticosteroid therapy per os and the
patient was discharged from hospital on day-8. Reviewed
one month later, the patient could walk without a cane but
was discreetly ataxic with an overall muscle strength of 4/5
in the lower limbs. The ophthalmology checkup reported a
clear improvement in vision.
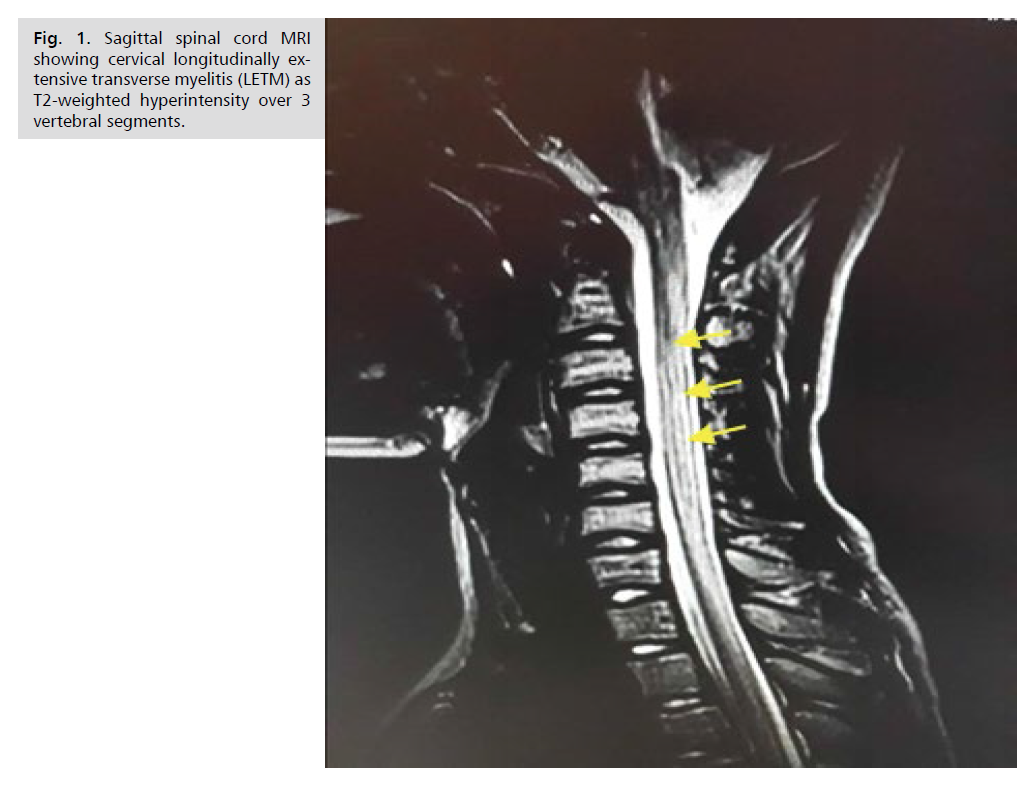
Figure 1: Sagittal spinal cord MRI showing cervical longitudinally extensive transverse myelitis (LETM) as T2-weighted hyperintensity over 3 vertebral segments.
Discussion
We reported Devic's disease in a 13-year-old female
patient who was initially followed for polyradiculoneuritis.
The particularity of our observation is the young age of the
patient. The median age of onset of this disease is 30 to 40
years, but pediatric or late onset, after 80 years, has been
reported [4]. NMO or Devic's disease is an autoimmune
disorder of the central nervous system (CNS), traditionally
restricted to the optic nerve and spinal cord. Like other demyelinating diseases of the CNS, the sex distribution
was strongly skewed toward female, with a female to
male ratio of 6.5:1 [4]. The patient we reported is female,
thus conforming to this female predominance. Myelitis
in NMO is classically presented as a longitudinally
extensive transverse myelitis (LETM) responsible for a
para/tetraplegia associated with a more or less symmetric
bilateral sensory deficit. This spinal cord syndrome is
associated with a sensory level and sphincter disorders [5].
All these characteristics of spinal cord injury were found in
our patient. However, the delay in diagnosis is related to
the absence of ophthalmological signs at the beginning of
the disease. This is supported by the authors who defined
Devic's disease as an association between myelitis and ON
[6]. It is also this association of myelopathy and visual
abnormalities that was reported in the Lancet in 1870
by Albutt [7]. Even if our patient did not present them,
it should be remembered that other clinical signs within
the framework of this disease exist and are well reported.
These include the area postrema syndrome, sleep disorders,
eating and thermoregulation disorders [6]. The diagnosis
of our patient was only evoked when visual disorders
appeared. The occurrence of this new symptom justified
the performance of MRI of the brain and spinal cord
associated with the search for antibodies. Indeed, the spinal
cord MRI confirmed extensive transverse myelitis, which is the most characteristic lesion of NMO [8]. The extensive
longitudinal character defining myelitis in NMO is a
radiological definition characterized by hyperintensity on
T2-weighted sequences that extends over more than three
contiguous spinal segments [6]. These abnormalities in
the spinal cord MRI have been reported to be, in general,
more frequently present in the cervical and the upper
thoracic spinal cord segments than the lower thoracic and
lumbar regions [9]. In our patient, the hyperintensity on
T2-weighted sequences spanned 6 spinal segments and
was located at the cervical level according to the literature
review. Although suggestive, MRI is not pathognomonic
[10], requiring other tests, in particular the search for
specific AQP-4 and anti-MOG antibodies to reinforce
the diagnosis that was evoked on MRI. Our patient had
a positive test of AQP-4 in her serum. In addition to
the clinical presentation and imaging, we confirmed the
diagnosis of NMO despite her young age. Studies have
reported the disease in young age populations. This is the
case of Cabrera-Gomez who reported in Cuba that the
onset of NMO before the age of 20 represented 3.4% of
all NMO cases [11]. More recently in 2020, Tenenbaum
et al. reported that the prevalence of NMOSD in children
represented 3-5% of all NMOSD cases [12]. Since the
discovery of anti AQP-4 antibodies and the numerous
publications reported in the last decades, NMO is now part of a group of pathologies called NMOSD and the
diagnostic criteria are continuously being modified, no
longer being limited to imaging or clinical or serological
criteria (Tab. 1) [13]. Untreated appropriately, NMO has
a rapidly deteriorating course leading to blindness and/
or major motor disability in half of the patients after an
average of five years [14]. Even when treated, the disease
can relapse, with an annual rate higher than in MS [8] of
which it was long considered a clinical form. Mortality in
NMO is high and most often occurs as a result of respiratory
distress related to bulbar extension of the cervical spinal
cord injury. As our patient's location on imaging was
cervical, appropriate treatment was undoubtedly decisive in
preventing bulbar extension in our patient. Currently there
is still no standard treatment protocol for NMO. However,
there are two main aspects to this treatment, namely the
management of relapse episodes and the prevention of
relapses [6]. In general, the management of acute episodes
is based on high dose intravenous corticosteroid therapy
with methylprednisolone at 1g/day for three to five days
followed by prolonged oral corticosteroid therapy [6]. The
particularity of this corticosteroid therapy in children is
the administration and adjustment of the dose according
to weight. It is 30 mg/kg/day of methylprednisolone
[15]. This dosage guided our choice of 840 mg per day
of intravenous methylprednisolone in our patient whose
weight was 28 kg. According to the literature review, other
treatments can be used in the acute phase. These are plasma exchange (5-7 cycles) [16] and the use of immunoglobulin.
Several molecules are proposed for relapse prevention
or background treatment of NMO. These include
azathioprine, rituximab, mycophenolate mofetil,
methotrexate, cyclophosphamide and mitoxantrone
[15,16]. Our patient received azathioprine 25 mg po every
12 hours with a good outcome.
| S. No |
Revised NMOSD Diagnostic Criteria |
| A |
NMOSD with AQP 4-IgG |
1. At least 1 core clinical characteristic |
| 2. Positive AQP4-IgG testing using best available method |
| 3. Exclusion of alternative diagnoses |
| B |
NMOSD without AQP4-IgG
or with unknown AQP4-
IgG status |
1. At least 2 Core clinical characteristics occurring as a result of
1 or more clinical attacks and meeting all of the following: |
a. At least 1 clinical characteristic: Must be optic neuritis,
LETM, or area postrema syndrome |
| b. Dissemination in space (2 or more |
c. Fulfillment of additional MRI requirements different core
clinical characteristics) |
2. Negative tests for AQP4-IgG using best available or testing
unavailable |
| 3. Exclusion of alternative diagnoses |
| C |
Core clinical characteristics |
1. Optic neuritis |
| 2. Acute myelitis |
| 3. Area postrema syndrome |
| 4. Acute brain stem syndrome |
5. Symptomatic narcolepsy or acute diencephalic syndrome
with typical diencephalic MRI lesions |
| 6. Symptomatic cerebral syndrome with typical brain lesions |
| D |
Additional MRI
requirements |
|
| i |
Acute optic neuritis |
a) Brain MRI normal or showing nonspecific white matter
lesions and |
b) Optic nerve MRI with T2-hyperintense or T1-weighted
gadolinium-enhancing lesion extending over >1/2 optic nerve
length or involving optic chiasm |
| ii |
Acute myelitis |
Requires intramedullary MRI lesion extending over ≥ 3
contiguous segments (LETM) or ≥ 3 contiguous segments of
spinal cord atrophy in patients with history compatible with
acute myelitis |
| iii |
Area postrema syndrome |
Requires associated dorsal medulla/area postrema lesions |
| iv |
Acute brainstem syndrome |
Requires associated periependymal brainstem lesions |
Abbreviations: AQP4: Aquaporin-4; IgG: Immunoglobulin G; LETM: Longitudinally Extensive
Transverse Myelitis; NMOSD: Neuromyelitis Optica Spectrum Disorder
Tab. 1. Revised NMOSD diagnostic criteria [13].
Conclusion
NMO, once rare not only in adults but even more so in
children, is becoming a common condition in recent years.
This is most likely related to a better understanding of the
pathophysiology and a definition of the related conditions
grouped as NMOSD. Although treatment is still under
discussion, high dose corticosteroid therapy in the acute
phase with or without plasma exchange or immunoglobulin
not only prevents fatal outcome but also allows recovery
with fewer sequelae. This condition should be evoked most
often in practice in the pediatric population taking into
account the diagnostic criteria even in the absence of AQP-4.
Acknowledgements
We do not have received any fund for the realization
of this article.
Conflicts of Interest
The authors declare that there is no conflict of interest.
REFERENCES
- Hor JY, Asgari N, Nakashima I, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol. 2020;11:501.
Google Scholar, Crossref, Indexed at
- Marignier R, Confavreux C. Neuro-optico-myélite aiguë de Devic et les syndromes neurologiques apparentés. La Presse Médicale. 2010;39(3):371-380.
Google Scholar, Crossref, Indexed at
- Vincent T. Neuromyélite optique et NMO-IgG. Revue Francophone des Laboratoires. 2008;404:21-23.
Crossref, Indexed at
- Mealy MA, Wingerchuk DM, Greenberg BM, et al. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69(9):1176-1180.
Google Scholar, Crossref, Indexed at
- Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28(1):105-120.
Google Scholar, Crossref, Indexed at
- Albutt TC. On the ophthalmoscopic signs of spinal disease. Lancet. 1870;95(2420):76-78.
Crossref
- Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurol. 2007;68(8):603-605.
Google Scholar, Crossref, Indexed at
- Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder. An international update. Neurol. 2015;84(11):1165-1173.
Google Scholar, Crossref, Indexed at
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol. 2014;27(3):279-289.
Google Scholar, Crossref, Indexed at
- Cabrera-Gomez JA, Kurtzke JF, González-Quevedo A, et al. An epidemiological study of neuromyelitis optica in Cuba. J Neurol. 2009;256(1):35-44.
Google Scholar, Crossref, Indexed at
- Tenembaum S, Yeh EA, Guthy-Jackson Foundation International Clinical Consortium (GJCF-ICC). Pediatric NMOSD: A review and position statement on approach to work-up and diagnosis. Front Pediatr. 2020;8:339.
Google Scholar, Crossref, Indexed at
- Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurol. 2015;85(2):177-189.
Google Scholar, Crossref, Indexed at
- Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurol. 1999;53(5):1107-1114.
Google Scholar, Crossref, Indexed at
- Tenembaum S, Chitnis T, Ness J, et al. Acute disseminated encephalomyelitis. Neurol. 2007;68(16 suppl 2):S23-S36.
Google Scholar
- Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord. 2012;1(4):180-187.
Google Scholar, Crossref, Indexed at
- Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the neuromyelitis optica study group (NEMOS). J Neurol. 2014;261(1):1-16.
Google Scholar, Crossref, Indexed at






