Zermeño-Rivera Jesús Jaime1, López-Martínez Carlos Horacio1, Fajardo-Barajas Daniel1, González-Jaime José de Jesús2 and Gutiérrez-Amavizca Bianca Ethel3
1Jalisco Plastic and Reconstructive Surgery Institute “Dr. José Guerrerosantos”, Eulogio Parra 2975 Interior 11, Prados Providencia, Guadalajara, Jalisco, Mexico
2Rehabilitation Electrodiagnosis Center and neurosurgical monitoring, Civil hospital of Guadalajara “Fray Antonio Alcalde”, Hospital 278, Guadalajara, Jalisco, México
3Department of Chemical and Biological Sciences, Institute of Biomedical Sciences, Autonomous University of the City of Juarez, Anillo Envolvente PRONAF and Estocolmo, City of Juarez, Chihuahua, México
Corresponding Author:
Gutiérrez- Amavizca Bianca Ethel
Department of Chemical and Biological Sciences
Institute of Biomedical Sciences
Autonomous University of the City of Juarez
Juarez city, Chihuahua, México.
Tel: +52 656 688 18 00; ext: 1551
E-mail: ethel90210@gmail.com
Received Date: September 26, 2016; Accepted Date: October 13, 2016; Published Date: October 18, 2016
Citation: Zermeño-Rivera JJ, López-Martínez CH, Fajardo-Barajas D, et al. Peripheral Retrograde Nerve Regeneration After Sciatic Nerve Injury in a Rat Model: A New Concept in Peripheral Nerve Surgery. J Univer Surg. 2016, 4:4. doi: 10.21767/2254-6758.100062
Background: Neuroregeneration after nerve injury occurs via anterograde (proximal in the distal) regrowth. The purpose of this study was to demonstrate that nerve regeneration occurs not only proximally to distally as classically described, but also distally to proximally using a rat model with a left sciatic nerve injury, with or without repair, compared to a healthy uninjured control group.
Methods: Fifteen rats were divided into 3 groups; a healthy control (Group 1, n=5), left sciatic nerve injury (Group 2, n=5), and left sciatic nerve injury and surgical retrograde nerve repair (Group 3, n=5). In Groups 2 and 3, complete surgical resection of the left sciatic nerve was performed and retrograde reconstruction of the nerve was done in Group 3 using an original, unique technique. Neuroconductive and histological variables were analyzed and compared between groups.
Results: After 15 weeks, Groups 1 (control) and 3 (retrograde reinnervation) behaved similarly, with no significant differences (p<0.05) in the proportion of Schwann cells and axons, amplitude waves, nerve conduction velocity, and latency response time (p<0.05), whereas Group 2 (injured unrepaired nerve) was significantly different from Groups 1 and 3 (p<0.05). The reconstruction of injured sciatic nerves showed histopathological and neuroconductive characteristics similar to that of the healthy control group 15 weeks after the reinnervation process.
Conclusion: This preclinical study demonstrates that retrograde nerve regeneration after sciatic nerve injury is feasible in a rat model 15 weeks after reconstructive nerve surgery. These results indicate that neuroregeneration is possible in a caudo-cephalic direction.
Keywords
Peripheral nerve regeneration; Retrograde; Sciatic nerve injury
Introduction
Neuroregeneration is a mechanism of repair and regrowth of nervous tissues, cells, or cell products after injury. The peripheral nervous system has an intrinsic ability for repair and regeneration. In contrast, repair of neurons in the central nervous system is very limited [1]. The capacity for regeneration is related to the age of the patient, mechanism of the injury, and the level of the injury (more distal injuries have better clinical outcomes) [2]. The pathophysiological mechanism of nerve regeneration remains unclear [3]. Damage to the spinal cord interrupts vital ascending and descending fiber tracts of spinally-projecting neurons. Because neuronal structures located proximal or distal to the injury site remain largely intact, a major goal of spinal cord injury research is to develop strategies to reestablish innervation lost as a consequence of injury [4].
The concept of retrograde peripheral reinnervation does not presently exist because so far the only known nerve growth is proximal to distal (anterograde peripheral reinnervation). The aim of this study was to demonstrate the possibility of retrograde peripheral nerve (caudal to cephalic) growth in a rat model with left sciatic nerve injury and retrograde repair reconnections in vivo, providing opportunities for new nerve transfers.
Methods
Fifteen specific-pathogen-free male Wistar rats (age 12 weeks; weight 240 g ± 10 g) were divided into 3 groups; a healthy control (Group 1, n=5), those with a left sciatic nerve injury (Group 2, n=5), and those with a sciatic nerve injury followed by surgical retrograde nerve repair (Group 3, n=5). The rats were housed in cages at 23°C ± 2°C, with good ventilation and natural day and night lighting, and free access to food and water. All rats were acclimated for one week before surgery. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by local authorities.
In Groups 2 and 3, complete surgical resection of the left sciatic nerve was performed. In Group 3, nerve reconstruction was done after the injury under a protocol of retrograde reinnervation outlet graft donor area. All surgeries were performed under anesthesia, and all efforts were made to minimize the pain and distress of the animals.
Retrograde surgical nerve repair technique protocol
We designed an original and unique technique for retrograde nerve reinnervation in a rat model as shown in (Figure 1).
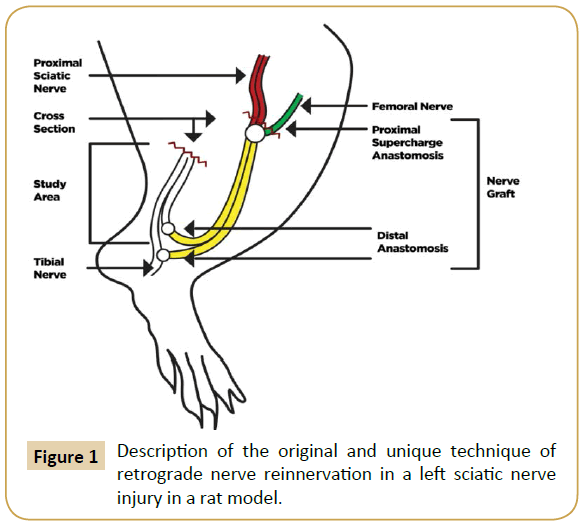
Figure 1: Description of the original and unique technique of retrograde nerve reinnervation in a left sciatic nerve injury in a rat model.
The donor area (right leg) was opened under an asepsis and antisepsis protocol, and the sciatic nerve identified as well as its division into the tibial and peroneal nerve branches. The nerve was taken as a Y-graft 1 cm in length from its location proximal to the distal branch, leaving the right leg without a sciatic nerve. The incision was then closed with nylon 4-0. Subsequently, the graft and the area receiving (left leg) the graft was opened and the left sciatic nerve was dissected, making a full neurotmesis in the proximal third. The point of the proximal neurorrhaphy of the left sciatic nerve was made in this site with the graft terminoterminal, as well as the supercharged termino-terminal with the femoral nerve, using 10-0 nylon. The two distal ends of the graft were anastomosed 1 cm below the section of the distal end of the division of the left sciatic nerve in the tibial and fibular nerves. One end was anastomosed termino-lateral to the tibial nerve, and the other end was anastomosed termino-terminal to the peroneal nerve. Leaving the sectioned segment of the proximal receptor of the tibial and peroneal nerves free to regenerate in a retrograde direction (study area) (Figure 2). All anastomoses were made with micro suture (Ethilon 10/0) prior to closing the incision.
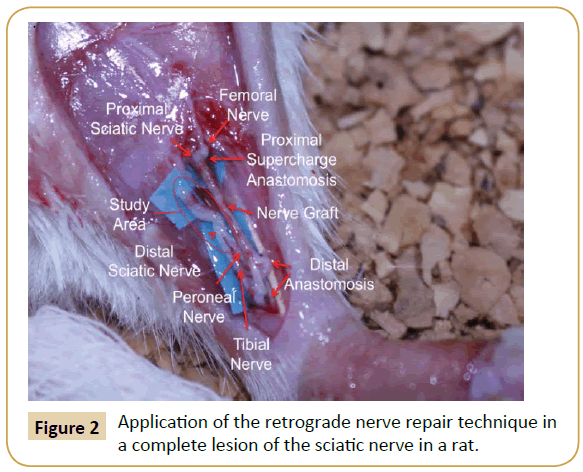
Figure 2: Application of the retrograde nerve repair technique in a complete lesion of the sciatic nerve in a rat.
After the operation, each rat was placed in its respective cage; later, the rats were moved to the care area where they were constantly monitored. They were fed and routinely cared for, taking special care of both hind legs.
Evaluation of nerve regeneration
Nerve conduction, histopathologic, and immunohistochemical studies of the sciatic nerves of the three groups were performed 15 weeks after the injury. Healthcare Vyasis model Vicking quest was used to study nerve conduction. Histological and immunohistochemical tests (hematoxylin-eosin, indigo blue, Masson trichrome, and Cajal staining as well as antibody staining against CD20, CD68, CD34, specific enolases, and S-100) were used on tissue samples from the sciatic nerves.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 statistical software, Excel 2010. Non-parametric tests were used and the differences between two continuous variables were determined using Kruskal Wallis test. Significance was established when p was less than 0.05, and confidence intervals were calculated at 95%.
Results
Fifteen rats were divided into 3 treatment groups and included in this study. Table 1 shows the average values of the variables analyzed by treatment group. Significant differences were found between Groups 1 and 3 versus 2 for proportion of Schwann cells and axons, amplitude wave, nerve conduction velocity, and latency response time (p<0.05).
| |
Group |
P valuea |
| 1 |
2 |
3 |
| Epineurium(thickness mµ) 10× |
32±16.43 |
28±13.03 |
56±40.37 |
0.411 |
| Perineurium (thicknessmµ) 10× |
10±6.12 |
5.4±2.88 |
6±2.23 |
0.306 |
| Endoneurium blood vessels(thickness, 10×) |
3.4±1.67 |
3.2±3.63 |
5.8±3.70 |
0.443 |
| Schwann cells (%) (40×) |
93±2.73 |
11±7.41 |
93±2.73 |
0.001* |
| Axons (%, 40×) |
94.4±0.89 |
3.6±1.34 |
91±2.23 |
0.000* |
| Mast cells (%) |
1.2±1.30 |
2.8±1.92 |
1±0.0 |
0.209 |
| Fibroblasts (%) |
1±2.23 |
2±4.47 |
2.2±4.38 |
0.908 |
| Wave amplitude (µV) |
1732.4±1918.38 |
0±0 |
355.4±337.33 |
0.000* |
| Nerve conduction velocity (ms) |
30.8±8.28 |
0±0 |
13.4±4.39 |
0.000* |
| Latency response time (ms) |
0.88±0.23 |
0±0 |
1.66±0.63 |
0.000* |
Table 1: Neuroconductive and histological variables analyzed in the three sciatic nerve groups.
Histological staining of the most representative nerves in each group is shown in Figure 3. Group 1 (Figure 3A) demonstrates the normal and healthy sciatic nerve structure, where normal cellularity is evident in all axons compared to Group 2 (Figure 3B), which clearly shows degenerated nerves with a loss of cellularity. Group 3 is represented in Figure 3C, showing a structure that is more similar to a normal nerve that the degenerated nerve in Group 2 (Figure 3B), with cellularity maintaining the nerve organization, epineurium, perineurium, and endoneurium.
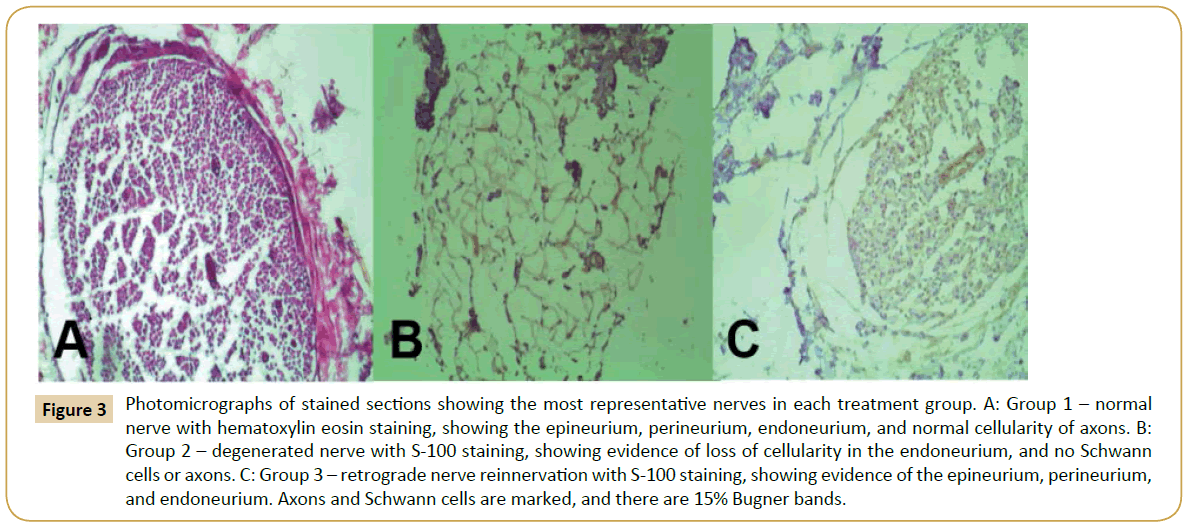
Figure 3: Photomicrographs of stained sections showing the most representative nerves in each treatment group. A: Group 1 – normal nerve with hematoxylin eosin staining, showing the epineurium, perineurium, endoneurium, and normal cellularity of axons. B: Group 2 – degenerated nerve with S-100 staining, showing evidence of loss of cellularity in the endoneurium, and no Schwann cells or axons. C: Group 3 – retrograde nerve reinnervation with S-100 staining, showing evidence of the epineurium, perineurium, and endoneurium. Axons and Schwann cells are marked, and there are 15% Bugner bands.
Discussion
The surgical technique that was performed in this study to allow for retrograde nerve regeneration consisted of developing a neurorrhaphy from a healthy donor nerve to a distal injured nerve segment, giving motor function to the proximal muscles in the area of the neurorrhaphy for nervous growth in a distalproximal direction.
On macroscopic histological examination, the sectioned sciatic nerve after surgical repair showed retrograde regeneration and reinnervation to adjacent muscle bundles (Figure 4).
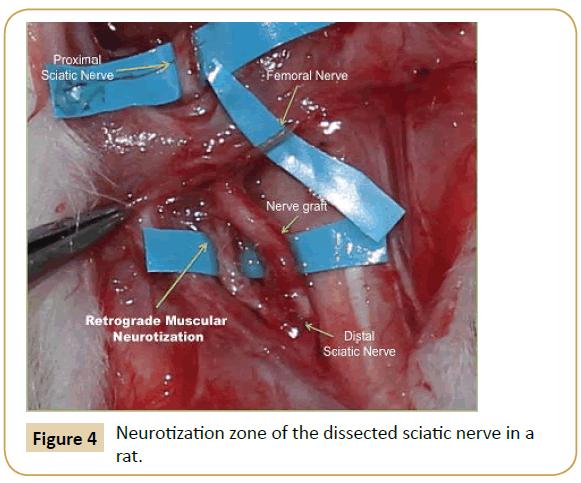
Figure 4: Neurotization zone of the dissected sciatic nerve in a rat.
In Group 1 with a healthy sciatic nerve, 94% Schwann cells, 1% fibroblasts, and other cells such as mast cells and capillaries were seen on the microscopic histological examination. On the reinnervated nerve, the histopathological analysis showed 91% Schwann cells, 2% fibroblasts, 1% mast cells, and 25% Bugner bands. Also observed was a thickened perineurium, an epineurium with abundant angiogenesis at the site of suture, and foreign body giant cells without granulomas, with an inflammatory infiltrate of lymphocytes. In the injured sciatic nerve, 5% Schwann cells were seen and the staining was negative for specific enolases. Additionally, almost 3% mast cells were found, which are involved in the inflammatory process, and angiogenesis was higher in the regenerated nerve compared to healthy nerves, although the difference was not significant, which is explained by the inflammatory process. All inflammatory processes initiate more blood vessels to repair the damaged tissue. By identifying the different dyes in reinnervated nervous tissue, the histopathological evidence in this study shows no significant intraneural inflammatory pattern when 10/0 sutures and the described technique (epi-perineural) were used. No histological changes were identified that could correspond to an acute inflammatory response. Cellularity corresponding to a chronic inflammatory response without forming granulomas was observed. Two or three Giant cells were located outside the endoneurium, which were enough to surround a 10/0 monofilament. Inside the endoneurium, macrophages and a few fibroblasts, identified by CD68 and CD34 staining, were found.
The neurostimulation study showed that the action potentials were similar in the reinnervated and healthy groups, but not in the degenerated nerves of Group 2.
The surgical technique used in this study for the reconstruction of the injured sciatic nerves showed histopathological and neuroconductive data that was similar to healthy nerves after 15 weeks of reinnervation. The study demonstrates that nerve regeneration in the caudo-cephalic direction may be possible, which has not been described and/or shown before.
One of the possible clinical applications of this new concept of "retrograde nerve regeneration" is to use new donor nerves, which are available from remote areas where the requirement for very long grafts limits reconstruction; openness to new nerve reconstruction techniques based on this principle, which allows for reinnervation in a caudo-cephalic direction; and to reach a proximal neuromuscular junction to innervate the same peripheral nerve in a distal direction.
It is also important to highlight that is the first study attempting to prove the theory of retrograde nerve regeneration, which has never been described in the literature. The limitations of this study are the small population size, evaluation of the variables over a short recovery time, and the absence of clinical evaluations. Additional studies are needed to confirm or refute these findings.
Conclusion
The principle of reinnervation in nerve reconstruction has so far been cephalocaudal. However, our study demonstrates neuroconductive recovery and histopathological evidence that retrograde peripheral reinnervation is possible in a rat experimental model, and the feasibility of rebuilding nerves in a caudo-cephalic direction.
Retrograde nerve reconstruction enables reinnervation to proximal muscles, generating a new concept of “retrograde reinnervation”. This technique provides a new option for surgical nerve reconstruction in peripheral nerves. It is a feasible option in nerve reconstruction for those cases where it is not possible to use proximal nerve reconstruction.
Acknowledgments
The authors would like to acknowledge Luis E Figuera of the Division of Genetics, Biomedical Research Center of the West, IMSS, Guadalajara, Mexico, for his contribution to the general supervision of the research group. This study received no financial support.
17366
References
- Palfai TP, Monti PM, Ostafin B, Hutchison K (2000) Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychol 109: 96-105.
- Harrison EL, Hinson RE, McKee SA (2009) Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav 34: 484-486.
- Howell A, Leyro T, Hogan J, Buckner J, Zvolensky M (2010) Anxiety sensitivity, distress tolerance, and discomfort intolerance in relation to coping and conformity motives for alcohol use and alcohol use problems among young adult drinkers. Addictive Behaviors 35:1144-1147.
- Krukowski RA, Solomon LJ, Naud S (2005) Triggers of heavier and lighter cigarette smoking in college students. J Behav Med 28: 335-345.
- Reed MB, Wang R, Shillington AM, Clapp JD, Lange JE (2007) The relationship between alcohol use and cigarette smoking in a sample of undergraduate college students. Addictive Behaviors 32: 449-464.
- Hughes JR, Kalman D (2006) Do smokers with alcohol problems has more difficulty quitting? Drug Alcohol Depend 82: 91-102.
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, et al. (1996) Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA: Journal of the American Medical Association 275: 1097-1103.
- Lisha NE, Carmody TP2, Humfleet GL2, Delucchi KL2 (2014) Reciprocal effects of alcohol and nicotine in smoking cessation treatment studies. Addict Behav 39: 637-643.
- Taylor B, Rehm J (2006) When risk factors combine: The interaction between alcohol and smoking for aerodigestive cancer, coronary heart disease, and traffic and fire injury. Addictive Behaviors 31: 1522-1535.
- Jarvis CM, Hayman LL, Braun LT, Schwertz DW, Ferrans CE, et al. (2007) Cardiovascular risk factors and metabolic syndrome in alcohol- and nicotine-dependent men and women. J CardiovascNurs 22: 429-435.
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB (2004) A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol, 65: 681-691.
- Kodl M, Fu SS, Joseph AM (2006) Tobacco cessation treatment for alcohol-dependent smokers: when is the best time? Alcohol Res Health 29: 203-207.
- Fu S, Kodl M, Willenbring M, Nelson D, Nugent S, et al. (2008) Ethnic differences in alcohol treatment outcomes and the effect of concurrent smoking cessation treatment. Drug and Alcohol Dependence 92: 61-68.
- Holt LJ, Litt MD, Cooney NL (2012) Prospective analysis of early lapse to drinking and smoking among individuals in concurrent alcohol and tobacco treatment. Psychology of Addictive Behaviors 26:561-572.
- Centers for Disease Control and Prevention (CDC) (2009) Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep 58: 1227-1232.
- Irving LM, Seidner AL, Burling TA, Thomas RG, Brenner GF (1994) Drug and alcohol abuse inpatients' attitudes about smoking cessation. J Subst Abuse 6: 267-278.
- Macnee CL, Talsma A (1995) Development and testing of the barriers to cessation scale. Nurs Res 44: 214-219.
- Orleans CT, Rimer BK, Cristinzio S, Keintz MK, Fleisher L (1991) A national survey of older smokers: treatment needs of a growing population. Health Psychol 10: 343-351.
- Kristeller JL (1994) Treatment of hard-core, high-risk smokers using FDA approved pharmaceutical agents: An oral health team perspective. Health Values 18: 25-32.
- Asher MK, Martin RA, Rohsenow DJ, MacKinnon S, Traficante R, et al. (2003) Perceived barriers to quitting smoking among alcohol dependent patients in treatment. Journal of Substance Abuse Treatment 24: 169-174.
- Martin RA, Rohsenow DJ, MacKinnon SV, Abrams DB, Monti PM (2006) Correlates of motivation to quit smoking among alcohol dependent patients in residential treatment. Drug Alcohol Depend 83: 73-78.
- Marlatt GA, Gordon JR (1985) Relapse prevention. New York: Guilford Press.
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N (1985) Decisional balance measure for assessing and predicting smoking status. J PersSoc Psychol 48: 1279-1289.
- DiClemente CC, Prochaska JO (1982) Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addict Behav 7: 133-142.
- Curry SJ, Grothaus L, McBride C (1997) Reasons for quitting: intrinsic and extrinsic motivation for smoking cessation in a population-based sample of smokers. Addict Behav 22: 727-739.
- Baha M, Le Faou AL (2010) Smokers' reasons for quitting in an anti-smoking social context. Public Health 124: 225-231.
- Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH (1995) A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol 63: 1005-1014.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 88: 791-804.
- First MB, Williams JB, Spitzer RL, Gibbon M (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute.
- Brown RA, Lejuez CW, Kahler CW, Strong DR (2002) Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol 111: 180-185.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86: 1119-1127.
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF (1994) Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav 19: 33-39.
- Fagerstrom KO, Heatherton TF, Kozlowski LT (1990) Nicotine addiction and its assessment. Ear Nose Throat J 69: 763-765.
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, et al. (2007) Exposure to the taste of alcohol elicits activation of the mesocorticolimbicneurocircuitry. Neuropsychopharmacology 33: 1391-1401.
- Fleming MF, Barry KL, MacDonald R (1991) The alcohol use disorders identification test (AUDIT) in a college sample. Int J Addict 26: 1173-1185.
- Cherpitel CJ (1995) Analysis of cut points for screening instruments for alcohol problems in the emergency room. J Stud Alcohol 56: 695-700.
- Macnee CL, Talsma A (1995) Predictors of progress in smoking cessation. Public Health Nurs 12: 242-248.
- Curry S, Wagner EH, Grothaus LC (1990) Intrinsic and extrinsic motivation for smoking cessation. J Consult Clin Psychol 58: 310-316.
- McBride CM, Pollak KI, Bepler G, Lyna P, Lipkus IM, et al. (2001) Reasons for quitting smoking among low-income African American smokers. Health Psychol 20: 334-340.
- Bonn-Miller MO, Zvolensky MJ (2009) An evaluation of the nature of marijuana use and its motives among young adult active users. Am J Addict 18: 409-416.
- Buckner JD, Zvolensky MJ, Schmidt NB (2012) Cannabis-related impairment and social anxiety: the roles of gender and cannabis use motives. Addict Behav 37: 1294-1297.
- Agrawal A, Budney AJ, Lynskey MT (2012) The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107: 1221-1233.
- Degenhardt L, Hall W, Lynskey M (2001) The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend 64: 319-327.
- Cohen J, Cohen P (1983) Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum.
- Cohen J, Cohen P, West SG, Aiken LS (2003) Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ US: Lawrence Erlbaum Associates Publishers.
- Lipkus IM, Feaganes JR, Green JD, Sedikides C (2001) The Relationship Between Attitudinal Ambivalence and Desire to Quit Smoking Among College Smokers. Journal of Applied Social Psychology, 31: 113-133.
- Wilson SJ, Creswell KG, Sayette MA, Fiez JA (2013) Ambivalence about smoking and cue-elicited neural activity in quitting-motivated smokers faced with an opportunity to smoke. Addict Behav 38: 1541-1549.
- Festinger LA (1957) A theory of cognitive dissonance. Evanston, IL: Row, Peterson.
- Markowitz LJ (2000) Smoker's perceived self-exemption from health risks. Psi Chi Journal of Undergraduate Research 5: 119-124.
- Jamieson P, Romer D (2001)What do young people think they know about the risks of smoking? In P. Slovic (Ed.), Smoking: Risk, perception, and policy (pp. 51-63). Thousand Oaks, CA US: Sage Publications, Inc.
- Schane RE, Glantz SA, Ling PM (2009) Social smoking implications for public health, clinical practice, and intervention research. American Journal of Preventive Medicine 37: 124-131.
- DaniJA, De Biasi M (2001) Cellular mechanisms of nicotine addiction. PharmacolBiochemBehav 70: 439-446.
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445-1449.
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, et al. (2002) Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend 67: 185-191.
- Kerst WF, Waters AJ (2014)Attentional retraining administered in the field reduces smokers’ attentional bias and craving.
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F (2010) Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction 105: 279-287.
- Raupach T, West R, Brown J (2013) The most "successful" method for failing to quit smoking is unassisted cessation. Nicotine Tob Res 15: 748-749.
- Korte KJ, Capron DW, Zvolensky M, Schmidt NB (2013) The Fagerström test for nicotine dependence: do revisions in the item scoring enhance the psychometric properties? Addict Behav 38: 1757-1763.









