Introduction
|
| |
| Cochlospermaceae is a family of two main genera; Amoreuxia and Cochlospermum and 20 to 35 species of trees and shrubs. They occur widely throughout the tropical regions of the world and most species in this family are either mesophytic or xerophytic, growing primarily in drier climates [1]. |
| |
| The plant is mainly a shrub or a small tree; 2 - 2.5m high. They have young branches and leaves with peltate scales from seeds and rhizomes. It is a common weed of cultivated fields in both Guinea and Sudan savanna zones. It produces 3 to 5-celled capsules containing numerous seeds whose epidermal trichomes are cotton [2]. |
| |
| It is widespread in the tropical regions from Senegal to West Cameroun and into East Cameroun but is curiously absent in Malaysia. It also grows in the Northern part of Nigeria especially the Chambas of the Vogel peak area (Benue River valley, Taraba state). |
| |
| The Stem decoction is used in the treatment of menstrual disorders. The root decoction is used in Northern Sierra Leone for the treatment of Gonorrhoea [3]. The decoction of root has been found to be effective for the treatment of uncomplicated Plasmodium falciparum without any major side effects [4]. In some African countries for example, East Senegal and Ivory Coast it is a medicinal plant that its rhizomes and leaves are used to treat many diseases: Malaria, Hepatitis, Diabetes, Infertility, Tryponosomiasis and certain infections treated by traditional healers as diarrhoea, Sexual transmissible infections [5]. Previous investigations showed that an aqueous extract of Cochlospermum planchonii rhizomes prepared in the manner used by native medical practitioners in Northern Nigeria can treat jaundice and protect people from liver damage [6]. The Hausa tribe of Nigeria and some tribes in Northern Sierra Leone use the stem bark in the production of fibres for making strings and ropes. The Chambas people in Sokoto, Nigeria use the threaded seeds as beads. The Nupe-Fulani people of Nigeria and some tribes in Sudan use the root extract as a yellow dye and it is also used to make tattoos, inks, mordants and stains. The leaf infusion is believed to bestow magical protection on the Fulani people of Northern Nigeria. Some tribes in Lagos use the root in cooking soup when palm oil is not available [2]. |
| |
|
Plant Collection, Identification and Preparation
|
| |
| The rhizome of Cochlospermum planchonii was collected on the 15th of January, 2010 from Binchi village, Bassa Local Government Area, Plateau State, Nigeria. |
| |
| The plant was identified in the field using keys and description given in the Useful plants of West Tropical Africa [3]. The collection (FHJ 154) was confirmed at the herbarium, Federal College of Forestry, Jos. |
| |
| The rhizome of the plant was washed to remove sandy debris, dried under a shade, powdered and stored in an airtight container until required for use. |
| |
|
Extraction
|
| |
| The fresh rhizome of Cochlospermum planchonii was air dried, powdered, sieved and referred to as ‘powdered rhizome ’. The powdered rhizome (500g) was defatted exhaustively with petroleum ether (60- 80OC) in a soxhlet apparatus. The defatted marc was subsequently extracted with water. The extract was concentrated in-vacuo to produce a dark brown mass (50g) subsequently referred to as ‘aqueous extract’. |
| |
|
Macroscopical Examinations
|
| |
| The macroscopical features of the rhizome were described using the terms outlined by [7]. |
| |
|
Microscopical Examinations
|
| |
| The powder and transverse section of the rhizome were used for this study. Both qualitative and quantitative studies were carried out. |
| |
| Chemo- microscopical examinations were also carried out to detect the presence or absence of various chemical constituents such as tannins, lignin, starch, fats and oils, mucilage, cellulose, cutin, protein and calcium oxalate crystals [8]. |
| |
|
Quantitative Evaluations
|
| |
| The moisture content of the powdered rhizome was determined by the ‘Loss on Drying Method’ [8]. The ash value and the acid insoluble ash were determined using methods described in the British Pharmacopoeia[9]. The extractive values (water and alcohol) were determined using methods described by Brain and Turner [10]. |
| |
|
Phytochemical Screening
|
| |
| The water extract was subjected to phytochemical screening for the presence of chemical constituents such as alkaloids, saponins, flavonoids, tannins, cardiac glycosides, anthraquinones and carbohydrates according to standard procedure [7]. |
| |
|
Analgesic studies
|
| |
|
(Acetic acid- induced abdominal constriction in mice):
|
| |
| The method described by Koster [11] and Locke [12] were used. The Mice were grouped into 10 each containing 5 animals. Group 1 were given acetic acid (0.7%v/v in saline) intraperitoneally (IP). Group 2 and 3 were given paracetamol, 500mg and 1000mg Ip before Acetic acid injection. Group 4, 5, 6, 7, 8 and 9 (Test groups) were given the extract at doses of 50mg and 100mg 30 minutes before acetic acid injection. Group 10 were given 0.1ml Normal saline 1p. The number of abdominal constrictions produced in each group for the succeeding 5 minutes was counted and compared with the response of other groups. This was calculated as the percentage of inhibition of abdominal constrictions. |
| |
|
Anti- inflammatory test. (Formalin test in rats)
|
| |
| The method used for this test was similar to that described by Dubuisson and Dennis [13] and was modified by Tjolsen et al [14]. Adult Wistar rats were divided into 5 groups each containing 4 rats. Group 1, 2, and 3 received 100, 200 and 400 mg extract per kg body weight respectively. |
| |
| The 4th group served as control and was given distilled water (10ml/kg) equivalent to vehicle given with the extract, while the 5th group was given 4 mg morphine per kg body weight. All drugs and extracts were given by Ip route. 30 minutes later, 50 μl of a freshly prepared 2.5% solution of formalin was injected subcutaneously (sc) under the planter surface of the left hind paws of each rat. They were placed in an observation chamber and monitored for one hour. The severity of pain response was recorded for each rat on the following scale. |
| |
| 0 - rat walked or stood firmly on the injected paws. |
| |
| 1 - The injected paws was favoured or partially elevated |
| |
| 2 - The injected paws was clearly lifted off the floor |
| |
| 3 - The rat licked, chewed or shook the injected paws Each rat was placed in such a way as to ensure unobstructed view of the injected paw. |
| |
| Anti-nociceptive effect was determined in two phases. The early phase (phase 1) was recorded during the first 5 minutes while the late phase (phase 2) was recorded during the last 45 minutes with a 10 minute lag period in between both phases. |
| |
|
Acute Toxicity Studies
|
| |
| The Locke method [12] and Duffus modules [15] were used for this study. Doses of 500, 1000, 2000, 3000, 4000 and 5000 Mg/Kg body weight (bw) and 8 mice were used. Doses of 10,000 and 11,000 Mg/Kg bw and 3 mice were used orally. |
| |
|
Statistical Analysis
|
| |
| All values were expressed as Mean ± S.E.M (Standard Error of Mean). Statistical significance was determined using the student’s t-test. Values with P<0.05 were considered significant. |
| |
RESULTS
|
| |
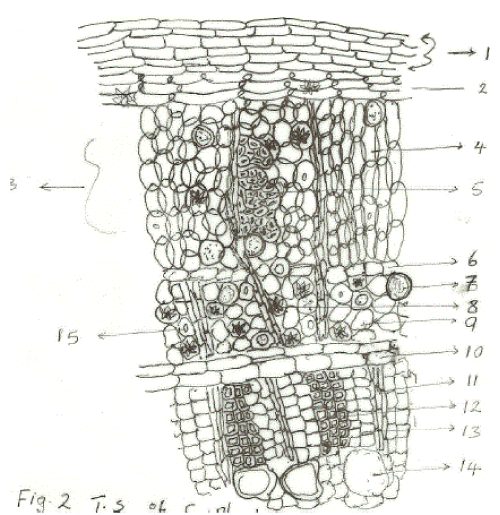
| The figure above shows the Transverse section of C. Planchonii with Six (6) layers of thin walled tabular, polygonal cells arranged in radial rows; with about 3 layers showing the phellogen. The cortex shows several layers of interlocked parenchymatous cells, collenchymatous cells with intercellular spaces, numerous oil cells with clusters of calcium oxalates. Commonly seen also are medullary rays of 2- 3 serrate running through the whole structure. The parenchymatous cells are spherical and contain starch grains, clusters of calcium oxalate and oil glands. The cambium is of about 1- 2 layers in tubular form and is located between the bark and wood layers which is arranged in radial rows. Thick walled sclereids are also present in the phelloderm with large piths. |
| |
DISCUSSION
|
| |
| Macroscopically, the rhizome has a cylindrical shape with root outlets. The texture is rough and woody while the colour of the rhizome is brown with a characteristic taste and odour. |
| |
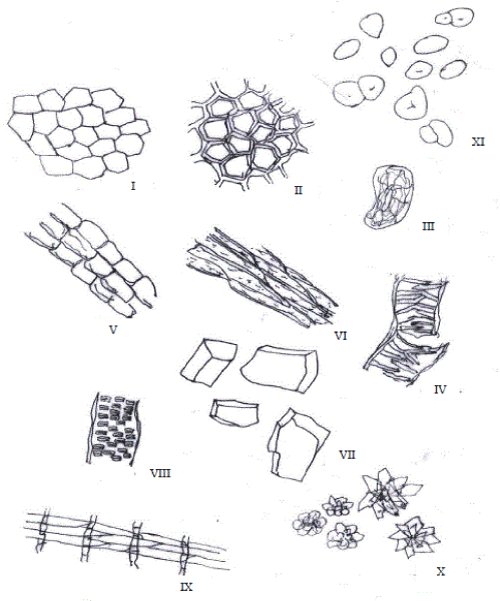
| Microscopically, double walled epidermal cells with straight walls were identified. Isolated sclereids also called stone cells were also seen. Clusters and rosettes of calcium oxalate crystals were found and are of considerable diagnostic importance in the examination of plant drugs and are also useful in some instances in distinguishing drugs of the same family [16]. |
| |
| Reticulate xylem and Sclenchymatous fibres were present, the starch grains occurred microscopically in various sizes (small, medium and large) and mainly spherical in shape without visible hilia and striations. These microscopic structures are most valuable in the identification of powdered drugs as their identification is largely based on the form, the presence or absence of certain cell types and cell inclusions [16]. Chemomicroscopical tests carried out revealed the presence of proteins, calcium oxalate crystals, lignin (in the epidermal walls, vessels and fibres), oils, tannins (prominently seen in the fibres) and starch grains (scattered all over). The starch grains occur microscopically as simple and compound granules that are abundant and of small, medium and large sizes mostly spherical shapes. |
| |
| The moisture content of the drug (7.20%) is not high thus it would discourage bacterial, fungal or yeast growth, as the general requirement for moisture content in crude drug should not exceed 14% [17]. |
| |
| Equally important in the evaluation of crude drugs is the total ash value. The total ash is particularly important in the evaluation of purity of crude drugs, i.e. the presence or absence of foreign inorganic matter such as metallic salts and/or silica [18]. |
| |
| The ethanol and water extractive value was found to be 2.91% and 3.92% respectively. Since the water extractive value was greater than that of alcohol, it means that water is a suitable extractive solvent than alcohol in the extraction of the rhizome powder of Cochlospermum planchonii. The alcohol and watersoluble extractives are indicators of the total solvent soluble component [19]. |
| |
| The phytochemical analysis of the extract revealed the presence of different chemical compounds such as alkaloids, glycosides, tannins and flavonoids among others were detected and would make the plant useful for treating different ailments and having a potential of providing useful drugs for human use. This is because the pharmacological activity of any plant is usually traced to a particular compound(s). The presence of tannins and other phenolic compounds, which have analgesic properties, could explain the use of this plant for the alleviation of pain and inflammation [7]. |
| |
| Acetic acid-induced nociception is usually used for the evaluation of mild peripheral analgesic drugs [20]. On the other hand, thermal painful stimuli are selective for the evaluation of centrally but not peripherally acting analgesic drugs [21]. |
| |
| In the acetic acid – induced writhing test in mice, the aqueous extract caused statistically significant (P<0.05) reduction in the mean number of writhes induced by acetic acid . Treatment with different doses (100-400mg/kg) of the extract significantly (P<0.05) reduced the number of writhing in a dose dependent manner. The number of writhes reduced from 35.25± 1.56, observed with the group administered distilled water to 10.75 ± 0.74 in the group administered 400mg/kg extract. The extracts were tested at three different dose levels (100,200 and 400 mg/kg), to know if they were dose dependent and there was significant differences in their analgesic activity hence they are dosedependent. The effect of the extract was quite comparable to that observed with Acetaminophen (paracetamol) which was used as a standard in acetic acid induced model. |
| |
| In the thermal hot plate test there was significant inhibition of central nociception at extract doses of 200 and 400mg/kg. However, the percentage pain inhibition was lower when compared with the standard used (Pentazocine). |
| |
| The results of the biological evaluation suggest that the aqueous extract may possess NSAID-like and opiod-like analgesic activities, mediated through both the peripheral and central mechanisms. However, results of the thermal (hot plate) experiment appear to suggest that the extract is more effective in alleviating peripheral pain (acetic acid) than central (hot plate) pain. |
| |
| Acetic acid induced writhing test has been associated with increase in the levels of prostaglandins E2 and F2 in peritoneal fluid [22]. The mechanism of activity of the extract may therefore be linked to cyclooxygenases and/or lipooxygenases. |
| |
| Analgesic and anti-inflammatory effects of flavonoids and tannins have been reported [23], hence the analgesic effects produced by the extract may be attributed individually or collectively to the flavonoids and tannins. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
 |
 |
 |
| Table 4 |
Table 5 |
Table 6 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








