Awajimijan Nathaniel Mbaba1, Michael Promise Ogolodom2*, Rufus Abam1, Mary-Jane Amadi1,
Robert Oziegbe Akhigbe3 and Abdulfatai Kolawole Bakre4
1Department of Radiology, Rivers State University Teaching Hospital, Port Harcourt Rivers State, Nigeria
2Rivers State Hospitals Management Board, Port Harcourt, Nigeria
3Rovina Medical Diagnostic Services, Lagos State, Nigeria
4Esteem Diagnostics Medical Services Ltd, Lagos State, Nigeria
- *Corresponding Author:
- Michael Promise Ogolodom
Rivers State Hospitals Management Board
Port Harcourt, Nigeria
Tel: +234803997393
E-mail: mpos2007@yahoo.com
Received date: 09 September 2019; Accepted date: 20 September 2019; Published date: 27 September 2019
Citation: Mbaba AN, Ogolodom MP, Abam R, Amadi MJ, Akhigbe RO, et al. (2019) Pituitary Adenoma, Multiple Sclerosis and Visual Impairment: How are they Related? A Case Report. Health Sci J Vol.13.No.5:679.
Keywords
Multiple sclerosis; Prolactin-secreting pituitary adenoma; Visual impairment
Introduction
Pituitary Adenoma and Multiple Sclerosis (MS) have been found in many patients and attempts to establish the relationship between them have drawn the attention of many researchers [1-5] and the definite correlation between MS and pituitary adenoma is still controversial [4].
Multiple Sclerosis attack triggered by hyperprolactinemia that is due to a prolactin-secreting pituitary adenoma has been reported [1]. MS is a chronic, persistent inflammatory demyelinating disease of the central nervous system (CNS), characterized pathologically by areas of inflammation, demyelination, axonal loss and gliosis scattered throughout the CNS [6]. Young adults are more commonly affected with an increased incidence in the colder wet temperate zones of the northern hemisphere [7]. The median age of MS onset is 30 years, but the disease can begin at any age [8]. It is twice as common in women as in men with a poorer prognosis in men. MS is a major cause of disability in Western countries in young adult population of Caucasian origin [6]. The presenting lesion usually affects the optic nerve (optic neuritis), spinal cord (acute transverse myelitis) brainstem (ophthalmoparesis) and cerebellum (clumsiness and gait ataxia). Visual impairment is common in multiple sclerosis (MS), and typical, remitting, unilateral optic neuritis frequently marks the onset of the disease [9]. Unusual progressive visual loss in MS has been reported by Read et al. [9].
A possible role of prolactin (PRL), a neuroendocrine peptide with powerful immunomodulatory properties is suggested in MS [1]. Prolactin is a hormone that is produced predominantly by the pituitary gland. The production of this hormone is increased in some adenomas of the pituitary. Pituitary adenomas are primary tumors of the pituitary gland and are one of the most common intracranial neoplasms. Pituitary tumours can be classified by size or by function [10]. The imaging modality of choice for the evaluation of pituitary adenomas is MRI [11]. On MR image, size less than 1 cm in diameter is considered a microadenoma and those tumours greater than 1 cm in diameter are considered a macroadenoma [10,12]. In laboratory investigation, function is described by the detectable increase of a pituitary hormone through blood tests and if hormone levels were normal, then tumour is defined as nonfunctioning [10].
Multiple Sclerosis has been described by several researchers as a predominantly immune-mediated demyelinating disease, as demonstrated by immune cell infiltration and accompanying inflammatory processes leading to damage of myelin. Although the etiology of MS is unknown, it has been attributed to genetic and environmental factors affecting the auto reactive immune responses [13,14]. It has been documented that hyperprolactinemia could be a possible trigger for multiple sclerosis. Several researchers have reported PRL as a disease-promoting factor in MS and Experimental Autoimmune Encephalomyelitis (EAE) (the animal model of MS) [3]. Prolactin might also promote CNS damage by breaking B cell tolerance [15-17], sustaining autoantibody (autoAb) production [18] and macrophage release of cytokines [19,20] and stimulating auto reactive T-cells [21,22]. Several immune cells involved in the autoimmune attack against the CNS occurring in MS and EAE can be stimulated by PRL [14].
Magnetic Resonance Imaging(MRI) is superior to Computed Tomography (CT) in the demonstration of MS plaques and it has been claimed that accuracy of nearly 100% can be obtained with modern machines with high magnetic field strength [6] MR images (T2W and FLAIR) show areas of high signal intensity in the periventricular white matter in 98% of MS patients. MS plaques are generally round to ovoid in shape. They are typically discrete and focal at the early stages of the disease, but become confluent as the disease progresses. The majority of MS patients have at least one ovoid periventricular lesion, whose major axis is oriented perpendicular to the outer surface of the lateral ventricles (Dawsons’ fingers) [6].
MS is the commonest white matter idiopathic inflammatorydemyelinating disease [6] and visual impairment resulting from a demyelinating process involving the optic nerve is one of the usual clinical manifestations of MS. MS is an uncommon disorder in the tropics and hyperprolactinemia may not be unconnected with this disorder in our setting.
Case Report
The patient is a 21-year old female who presented for brain MRI. She was referred for the study to exclude space occupying lesion following her unusual bilateral visual field impairment. The MRI scan revealed pituitary maroadenoma and multiple white matter lesions, which were diagnosed as multiple cerebral infarcts. However patient observed progressive worsening of her vision and was referred for a repeat brain MRI and pituitary hormone analysis after 9 months. Brain scan showed multiple widespread discrete T2W and FLAIR hyperintense and T1W isointense (with brain) lesions predominantly in the deep cerebral and cerebellar white matter (Figures 1 and 2). The long axis of one of which is perpendicular to the lateral ventricle (Dawsons’ fingers) (Figure 1). Some of these lesions show mild enhancement with contrast in keeping with Multiple Sclerosis. The pituitary gland is mildly enlarged with marked enhancement. It measures 1.26 cm in its sagittal diameter consistent with a maroadenoma (Figure 3). No mass effect on the optic chiasma and cavernous sinuses. The repeat study was compared with the previous one and it revealed increase in the number of white matter lesions while the pituitary lesion showed no difference in size. Pituitary hormone laboratory study showed marked increase in Prolactin level: 40.5 ng/ml (N=1.2-20 ng/ml). The overall findings are suggestive of Multiple Sclerosis possibly triggered by Hyperprolactinemia from pituitary adenoma.
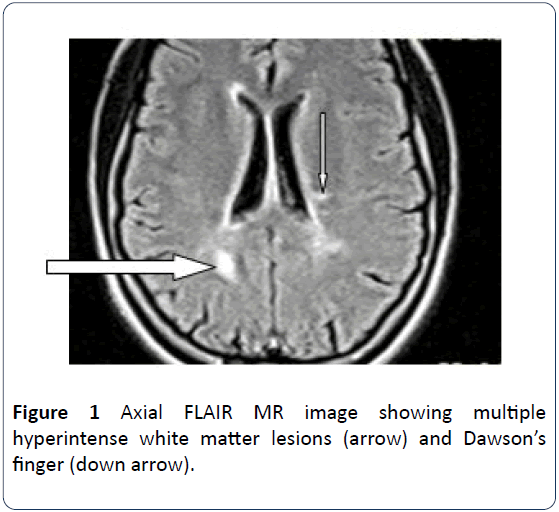
Figure 1: Axial FLAIR MR image showing multiple hyperintense white matter lesions (arrow) and Dawson’s finger (down arrow).
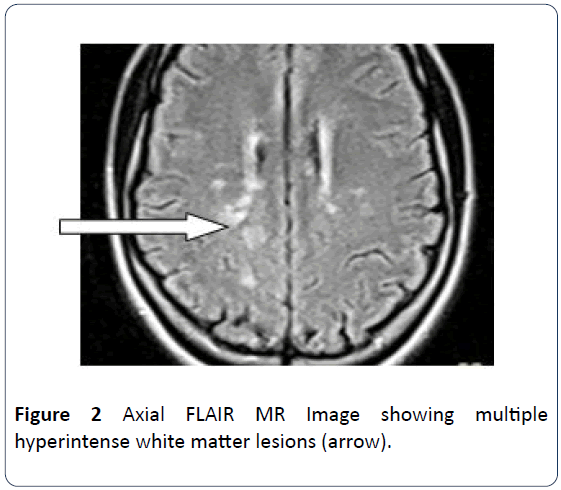
Figure 2: Axial FLAIR MR Image showing multiple hyperintense white matter lesions (arrow).
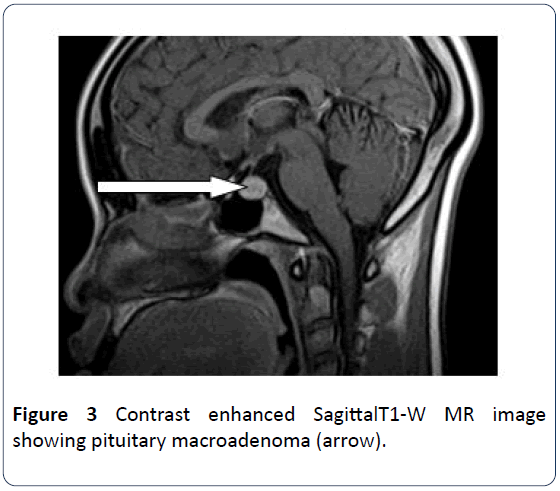
Figure 3: Contrast enhanced SagittalT1-W MR image showing pituitary macroadenoma (arrow).
Discussion
Prolactin is recently known to play an important role in some autoimmune diseases such as systemic lupus erythematous (SLE), rheumatoid arthritis (RA), Sjogren’s syndrome (SS), Hashimoto’s thyroiditis (HT), Celiac diseas e (CD), Graves’ disease (GD), lymphocytic hypophysitis (LH), Addison’s disease (AD), diabetes mellitus (DM) type I, and MS [4,23-25]. Prolactin belongs to the growth and lactogenic hormone family and has potent immunomodulating properties. Mild hyperprolactinemia has been found to enhance several autoimmune diseases and increased PRL plasma levels have been described in the experimental multiple sclerosis (MS) model while the PRL antagonist bromocriptine was able to suppress the disease [2].
In this study, prolactin secreting pituitary macroadenomas and MS associated with visual impairment were detected in the patient under review. This is in keeping with the report of the study conducted by Nociti et al. [1]. In their study, they documented the case of a 32-year-old MS patient who developed the irst white matter lesions in association with the development of a PRL-secreting adenoma, which approximately doubled the serum PRL levels. The adenoma was excised, but 12 years later the patient suffered two additional disease attacks, in concomitance with adenoma recurrence. Consequently, they concluded that prolactin may have facilitated the in lammatory process and triggered MS clinical attacks, suggesting a role of prolactin in immunomodulation and therefore in autoimmune disease course. In a recent work, the effect of PRL on B cells isolated from MS patients has been explored [3,26]. PRL signi icantly enhances the number of cells secreting antibodies directed against myelin oligodendrocyte glycoprotein (MOG) in MS subjects and up regulates the expression of B cell activating factor (BAFF) and the anti-apoptotic molecule Bcl2, via a Jak2/ STAT-dependent pathway.
Our patient is a young woman in whom MS and a prolactin secreting pituitary adenoma were identi ied following a complaint of unusual bilateral progressive visual impairment. In parallel to our report, Read et al. [9] in their study on progressive visual loss as an unusual presentation of multiple sclerosis reported three cases of patients who presented with progressive visual loss and in whom MS was diagnosed a ter other causes of progressive visual loss were excluded. All the patients had lesions consistent with MS outside the visual pathways on magnetic resonance imaging. Ormerod and McDonald [27] also reported 5 cases of progressive visual failure in patients in whom MS was subsequently diagnosed a ter other potential causes had been excluded. Although other potential causes of visual loss need to be excluded, Read et al. [9] and Ormerod and McDonald [27] concluded that multiple sclerosis may infrequently present with progressive visual loss and must be considered in the differential diagnosis in this setting.
Predominance of women in autoimmune diseases suggests that sex hormones may play a role in disease susceptibility. PRL is a lactogenic hormone that may play a role in the pathophysiology of MS. MS is relatively common in Europe, the United States, Canada, New Zealand and parts of Australia, but rare in Asia, and in the tropics and subtropics of all continents [6]. Giving the fact that MS is relatively an infrequent disorder in the tropics, and MS and prolactin secreting adenoma were diagnosed in our patient, the role of hyperprolactinemia in the occurrence of multiple sclerosis in this patient could be suggested. This supposition has also been made by a host of researchers [1,3,14].
Although there is no consensus on the association between MS and hyperprolactinemia [28], reports abound in the literature on the possible role of PRL in the pathogenesis of MS. Based on preliminary evidence that hormonal changes may appear before the symptomatic phase of the disease [29,30]. It is tempting to speculate that a pro-inflammatory hormone favors the rupture of tolerance, which is a key feature of autoimmunity [14].
Conclusion
The relationship between hyperprolactinemia and MS is still being investigated. No consensus has been reached. The discovery of a rare disease, in the tropical region in a patient in whom prolactin secreting adenoma was also diagnosed calls for further studies to properly define the relationship between hyperprolactinemia and multiple sclerosis. Clinicians, surgeons, ophthalmologists and radiologists need to remember this association when considering the differential diagnosis of a patient presenting with unusual visual impairment when other potential causes must have been excluded.
24700
References
- Nociti V, Frisullo G, Tartaglione T, Patanella AK, Iorio R, et al. (2010) Multiple sclerosis attacks triggered by hyperprolactinemia. J Neurooncol 98: 407-409.
- Haseen C, Gold SM, Bruhn M, Monch A, Schulz K (2002) Prolactin stimulation in multiple sclerosis – An indicator of disease subtypes and activity? Endocrine research 28: 1-2.
- Costanza M, Pedotti R (2016) Prolactin: Friend or Foe in Central Nervous System Autoimmune Inflammation? Int J Mol Sci 7: 12-15.
- Zadeh AR, Farrokhi M, Etemadifar M, Beni AA, Dastravan N (2015) Prevalence of benign tumors among patients with multiple sclerosis. Am J Exp Clin Res 2: 127-132.
- Ioyleva E, Makarenko I (2017) Visual impairment in combined pathology: multiple sclerosis and pituitary adenoma. Abstracts from the 2017 European Association for Vision and Eye Research Conference 95: S259-265.
- Grainger and Allison’s Diagnostic Radiology-A textbook of medical imaging. (6th edn.). 2: 1514-1521.
- Sutton D (2002) Textbook of Radiology and imaging, (7th edn.). 2: 1799-1800.
- Zhornitsky S, Yong VW, Weiss S, Metz LM (2012) Prolactin in multiple sclerosis. Mult Scler Journal 19: 15-23.
- Read SJ, Harrison JD, Pender MP (1996) Progressive visual loss: An unusual presentation of multiple sclerosis. J Clin Neurosci 3: 264-267.
- Saunders S, Vora JP (2008) Endocrine evaluation of pituitary tumours. Br J Neurosurg 22: 602-608.
- Dekkers OM, Pereira AM, Romijn JA (2008) Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab 93: 3717-3726.
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, et al. (2004) The prevalence of pituitary adenomas: a systematic review. Cancer 101: 613-619.
- Ebers GC (2008) Environmental factors and multiple sclerosis. The Lancet Neurol 7: 268-277.
- Deckx N, Lee W, Berneman ZN, Cools N (2013) Neuroendocrine immunoregulation in multiple sclerosis. Clin Develop Immunol 70: 1-23.
- McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE (1991) Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol 147: 3780-3787.
- McMurray R, Keisler D, Izui S, Walker SE (1994) Hyperprolactinemia in male NZB/NZW (B/W) F1 mice: Accelerated autoimmune disease with normal circulating testosterone. Clin. Immunol. Immunopathol 71: 338-343.
- Peeva E, Michael D, Cleary J, Rice J, Chen X, et al. (2003) Prolactin modulates the naive B cell repertoire. J Clin Investig 111: 275-283.
- Peeva E, Gonzalez J, Hicks R, Diamond B (2006) Cutting edge: Lupus susceptibility interval SLE3/5 confers responsiveness to prolactin in C57BL/6 mice. J Immunol 177: 1401-1405.
- Majumder B, Biswas R, Chattopadhyay U (2002) Prolactin regulates antitumor immune response through induction of tumoricidal macrophages and release of IL-12. Int J Cancer 97: 493-500.
- Malaguarnera L, Musumeci M, Licata F, Di Rosa M, Messina A, et al. (2004) Prolactin induces chitotriosidase gene expression in human monocyte-derived macrophages. Immunol Lett 94: 57-63.
- Costanza M, Musio S, Abou-Hamdan M, Binart N, Pedotti R (2013) Prolactin is not required for the development of severe chronic experimental autoimmune encephalomyelitis. J Immunol 191: 2082–2088.
- Legorreta-Haquet MV, Chavez-Rueda K, Montoya-Diaz E, Arriaga-Pizano L, Silva-Garcia R, et al. (2012) Prolactin down-regulates CD4+CD25highCD127low/regulatory T cell function in humans. J Mol Endocrinol 48: 77-85.
- Shelly S, Boaz M, Orbach H (2012) Prolactin and autoimmunity. Autoimmun Rev 11: A465-470.
- Peeva E, Venkatesh J, Michael D, Diamond B (2004) Prolactin as a modulator of B cell function: implications for SLE. Biomed Pharmacother 58: 310-319.
- Etemadifar M, Najafi MA, Najafi MR, Alavi A, Nasr Z, et al. (2014) Multiple sclerosis and hyperprolactinemia: a case-control study. Acta Neurol Belg 115: 253-257.
- Correale J, Farez MF, Ysrraelit MC (2014) Role of prolactin in B cell regulation in multiple sclerosis. J Neuroimmunol 269: 76-86.
- Ormerod IEC, McDonald WI (1984) Multiple sclerosis presenting with progressive visual failure. J Neurol Neurosurg Psychiatry 47: 943-946.
- Shelly S, Boaz M, Orbach H (2012) Prolactin and autoimmunity. Autoimmun Rev 11: A465-A470.
- Walker SE, Jacobson JD (2000) Roles of prolactin and gonadotropin-releasing hormone in rheumatic diseases. Rheum Dis Clin North Am 26: 713-736,.
- Peeva E, Zouali M (2005) Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol Letters 101: 123-143.








